Comparative studies on role of synthetic and biologically derived surface-active agents on removal of oil from oil spilled soil
Abstract
The present studies highlight the isolation, screening, production and evaluation of bioremediation efficacy of marine microbial surface-active agent on crude oil contaminations. Based upon screening, six isolates were confirmed as capable of producing surface active agents were obtained from marine sediments of Ennore harbor, Chennai, Tamil Nadu and these isolates were identified as bacillus genera with significant differences in their morphology. For the maximum production of surface-active agents, nutritional optimization in 6 screened isolates revealed that sucrose and yeast extract were the suitable carbon and nitrogen sources for growth in pH of 7.2 ± 0.2, at 37°C temperature and agitation at 180-200 rpm. Extraction and characterization studies revealed, the product was polymeric in nature with surface activity (28 ± 4 mN/m), thermal and emulsification stability (more than 90 days) when compared with synthetic surfactants. Based on laboratory scale studies, 90% of crude oil was removed from the aqueous phase within 60-120 minutes of exposure to the partially purified surface-active agent. This study suggested that the Bacillus species can be utilized for cleaning up hydrocarbon contamination sites and concurrently produce bio surfactants.
Keywords
Bioremediation; Crude oil removal; Biosurfactant; Surface tension; Oil contaminated soil
Introduction
Soil contamination by oil-based goods has become one of the principal issues because the synthetics and perilous materials they contain are a wellspring of worry for the whole ecosystem, so it is vital to track down an answer to this issue [1]. Oil is one of the main wellsprings of energy in the cutting edge modern world. However long the oil is presently double-dealing, transport, stockpiling and use, there is a gamble of spillage. Oil slicks represent a serious natural issue [2]. On 28 January 2017, an oil slick happened at Kamarajar Port in Chennai, Tamil Nadu. Despite the fact that legislatures and NGOs have gone to essential lengths to safeguard human existence, the effect has not been totally wiped out. Various methods have been developed to remove oil from contaminated areas. Mechanical lifting over oil using sorbents is certain of the close pregnant counter measures. Oil spills the oceans are over a massive situation due in imitation of their poor monetary or ecological impacts [3].
Oil spills posture severe threats to coastal ecosystems, ranging beyond instantaneous monetary losses in imitation of long-term negative results on the interactions between ecological elements [1]. This is because of its densely populated formal [4]. On a world estimate into 1970-2015, 238 marine dark lantern spill incidents have befell shut in conformity with coastal environments [5], affecting tremendous sky over vegetation [6], sand seashore [7], and marine mammals than birds [8].
Therefore, to the extent that a new coastal decision support system considers environmental conditions, it is necessary to rapidly identify vulnerable areas or resources. Oil spills and their impact on this environment will reach and affect specific aspects of interest within the study area. Thus, the study addresses this gap by adopting the following model and predicts an oil slick path in the study area. In the present study, it resulted in the formation of biosurfactants or herbal surfactants similar to bacteria as a result of microorganisms. Biosurfactants have similar properties to surfactants from chemical sources. For example, biosurfactants produced by Bacillus species have been used to remove crude oil from oil-contaminated (leaked) soils.
Materials and Methods
Screening of bio surfactants producing microorganism
Two marine sediment samples were collected from marina beach, Tamil Nadu, India. According to the procedures summarized by Saravanan et al., [9]. Zobell marine broth and agar (for bacteria) were the media used for the isolation of microbial species according to the standard procedure employed for the cultivation and maintenance of marine organisms. Morphologically distinct microbial colonies are screened and classified based on their Gram’s reaction. All the pure cultures were stored at -80°C in the presence of 30% of glycerol. For screening of biosurfactants producing organisms, all the obtained pure cultures were grown in Zobell medium individually at 37°C under 150 rpm for 48 hours. The biosurfactants activity of the cell-free medium was assessed according to the methods of Tugrul and Cansunar [10].
Production and extraction of biosurfactants
Bacillus sp., was inoculated in Zobell marine broth (100 ml in 1000 ml capacity conical flask) at 37°C for 24-48 hours under shaking conditions. Followed by incubation, the samples were made cell-free by centrifugation at 10,000 rpm at 4°C and the cell-free supernatant was mixed with an equal amount of ice-cold ethanol and kept at 4°C for overnight. The resultant pellet was obtained based upon centrifugation and lyophilisation considered a partially purified biosurfactant. The partially purified biosurfactant was dissolved in water and subjected to physical, chemical, surfactant activity and instrumental characteristics.
Surfactant activity drop collapse test
The Surface activity of biosurfactant was confirmed by drop-collapse test according to the procedure described by Tugrul and Cansunar [10]. A drop of biosurfactant solution was added to the center of an oil drop (20 µl of any oil) taken in clean glass slide. The collapse of oil drop has been visualized and less time taken indicates higher the activity of a surfactant.
Surface activity measurements
Surface tension of cell-free broth was measured by ring method using GBX-3S tensiometer (DM) at room temperature. A 10 ml of sample was taken in a clean glass beaker and placed on tensiometer platform. A sterile platinum ring was submerged into solution and then slowly pulled through the liquid-air interface. Each result on surface activity of the cell-free broth was measured as mN/m and the average of 10 determinations after stabilization. Critical Micelle Concentration (CMC) obtained surface active agents were calculated [11].
Removal of oil in aqueous and soil
Optimization of cell and oil concentrations for the oil removal: Growth medium of 50 ml sterile Zobell broth in 500 ml flask having different cell concentrations 2, 20, 200 and 2000 µl of stock culture containing 8, 80, 800 and 8000 × 104 of cells respectively, various amounts of oil was added (0.5, 1, 2.5, 5, 7.5 and 10 ml) to different concentration of cell culture and it was incubated individually for 48 hours in an orbital shaker at 37°C. After 48 hours, the flask culture was centrifuged and the supernatant was subjected to oil removal analysis.
Preparation of oil contaminated soil
Different types of soil (sand, beach, clay and clay loamy) were collected. After sampling, the soil was completely air-dried and sieved on a 10-mesh (<2 mm) and was screened prior to use. The sieved soil sample was analyzed for particles size distribution and soil texture was sandy soil, beach soil, clay soil and clay loamy soil. The soil was autoclaved three times to avoid the other bacterial contamination. In a 1 L beaker, 100 g of air-dried soil was added and 2.5 ml of crude oil was mixed evenly. These steps were repeated for 4 times until the approximate concentration of crude oil in the soil was 10 ml/100 g of soil. The crude oil contaminated soil was placed in fume hood for 7-10 days to oil adsorption in the soil.
In-situ removal of crude oil from contaminated soil
Growth medium of 50 ml sterile Zobell broth and 10 g of contaminated soil (containing 1 g of oil) was taken in 500 ml sterile flask and inoculated with 80 × 104 (20 µl) of cells and incubated individually for 48 hours in orbital shaker at 37°C. After 48 hours, flask culture was centrifuged and the supernatant was subjected to oil removal analysis.
Oil contaminated soil washing with cell free supernatant and partially purified bio surfactant
The soil washing study was conducted to observe crude oil removal with the cell free supernatant and partially purified biosurfactant. 10 g of soil mixed with 50 ml of cell free supernatant and partially purified biosurfactant individually. Every 30 mins the aqueous part was removed and subjected to analysis.
Comparison of synthetic surfactants and bio surfactant for the removal of crude oil
In order to determine the removal of crude oil, the surfactants such as SDS, Tween 80, triton-X 100, Cetyl Trimethyl Ammonium Bromide (CTAB), phospholipid and biosurfactant were isolated from marine Bacillus sp and was taken for the comparison studies for the removal of crude oil. Three different concentrations (1, 5 and 10%) of surfactants and biosurfactants were carried out for the oil removal study.
Shaking and static condition
Different concentration of surfactant (both synthetic and bio-) were taken in the 500 ml of flask containing 1 gm contaminated soil and incubated individually under shaking (200 rpm) condition. Every 30 mins, they were centrifuged, subsequently aqueous and soil were separated which was mixed with hexane (1:1) and it was subjected to separation technique for the oil removal.
Different concentrations (%) of surfactant (both synthetic and bio-) were taken in the 500 ml of flask which contains 1 gm contaminated soil and was incubated under static (200 rpm) condition individually.
The flask was centrifuged at 200 rpm for 6, 12, 24 and 48 hours and separated aqueous and soil, the residual oil was (extracted with hexane) subjected for the removal analysis.
Determination of oil removal study
The oil removal was determined by solvent extraction using n-Hexane. Equal amount of n-Hexane was added to the separated aqueous samples and then shaken laterally. The hexane portion was separated and repeated thrice. The entire extract sample was collected and the absorbance of the extract samples were measured at 420 nm using a stock solution of n-hexane/crude oil mixture, which showed the highest absorbance occurred at 420 nm.
The concentration of crude oil at this absorbance was determined from the function obtained from the calibration curve of the stock solution n-hexane/crude oil at 200°C. The percentage of crude oil removal from the water and soil was calculated using the equation.
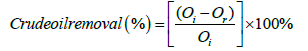
Oi is the initial oil in the soil (g)/water before washing and Or is the oil remaining in the soil (g)/water after washing.
Determination of oil removal by FT-IR spectral analysis
Biosurfactants samples mixed with KBr (Sigma, US) and the pellet obtained after hydrolytic pressure was analyzed using Spectrum one (Perkin-Elmer Co., USA model). All measurement consists of 500 scans and plain KBr pellet was used as the background reference.
Results and Discussion
Before Bacillus sp., was isolated from marine sediment and it was sub cultured in Zobell marine broth and stored in 30% glycerol stock for future use. Morphology of Bacillus sp. has rough, dry, colonies. Gram positive rode of length >160 µm and breath of 400 nm observed under scanning electron microscope (Figures 1a and 1b).
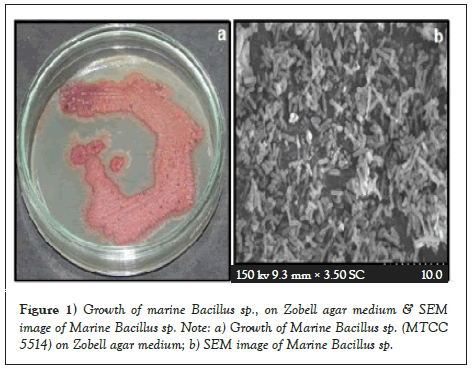
Figure 1: Growth of marine Bacillus sp., on Zobell agar medium & SEM image of Marine Bacillus sp. Note: a) Growth of Marine Bacillus sp. (MTCC 5514) on Zobell agar medium; b) SEM image of Marine Bacillus sp.
Several researchers have isolated biosurfactant produced strains belonged to 8 different genera, i.e. Bacillus, Rhodococcus, Halomonas, Alcanivorax, Exiguobacterium, Halomonas, Pseudomonas and Streptomyces. Among them, three were recently established, i.e., Alcanivorax, Exiguobacterium and Halomonas. The genus Alcanivorax is particularly young and was established in 1998 [12], with only 11 species identified so far. All of them were isolated from marine environments and were found to be important alkane degrading bacteria [13], India [14], Iran [15] and Germany [16], most of which are from inland reservoirs. The reported biosurfactant producers in reservoirs included Bacillus licheniformis strains [16,17], Bacillus cereus strain [17], Bacillus subtilis strains [18,19]. Those biosurfactant producers identified from oil field samples were confirmed as the genera of Bacillus sp.
Instrumental analysis of extracted sample
Crude oil and crude oil transformed samples were subjected to UV-visible spectroscopy, FT-IR and GC analysis. Bacillus sp was isolated from marine sediment and it was subcultured in Zobell marine broth and stored in 30% glycerol stock for further use. Morphology of Bacillus sp has rough, dry, filamentous with pink pigmented colonies. Gram positive rods of length >150 μm and breadth of >400 nm observed under scanning electron microscope. The isolate follows typical growth pattern and the stationary phase achieved only after 180 minutes of incubation.
During growth phase there was increase in the media pH and the final pH was observed as >8.5 ± 0.4. The qualitative activity was analyzed using the drop collapse test which showed that the oil was collapsed immediately when placed on 10 µl of surfactant solution Surface activity measurements made during the growth of the isolate exhibit surfactant activity after 24 hours of incubation and the maximum surfactant activity observed after 48 hours and maintained till 72 hours. The maximum surface activity of the marine isolated was measured 28 ± 5 mN/m. CMC is a direct indication of the surface activity of a surfactant product. The lower the CMC, the smaller quantity of the surfactant is needed to reach the micellar stage, thus the greater the associated surface activity [20].
Figure 2 illustrated that the bioreactor in single batch which contains 1600 ml of zobell marine broth which was prepared under aseptic condition. 2 ml of the inncolum was added to the sterile media. The growth was observed after 48 hours interval with reference to the yield of the biosurfactant. The ethanol precipiataion procedure was followed which exhibits 35-40 g/L (wet weight) of the surfactant and after lyophilization and drying 4-6 g/l of biosurfactant was obtained. The surface activity, which result in a broad spectrum of potential applications in oil contaminate control (Figure 2) [21].
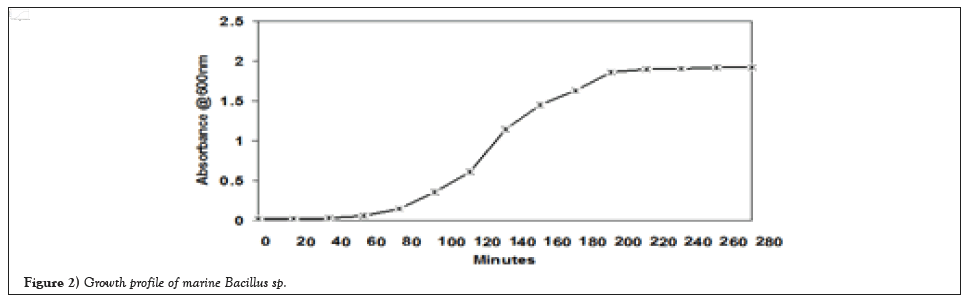
Figure 2: Growth profile of marine Bacillus sp.
Figure 3 illustrated surface tension plot obtained from log concentration of biosurfactants versus surface tension measured in mN/m. The critical micellar concentration was obtained at 2.301 log concentration of biosurfactants (Figure 3).
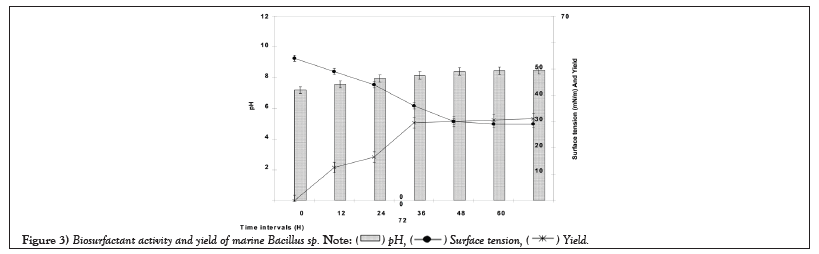
Figure 3: Biosurfactant activity and yield of marine Bacillus sp. 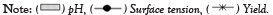
With respect to the optimization of cell concentration, different concentrations of cells were inoculated in 50 ml of broth with 10 ml of oil were given and incubated individually for 48 hours. In which the 20 µl (80 × 104 cells) is the minimum concentration required for the removal/solubilization of crude oil in aqueous medium (Figure 4).
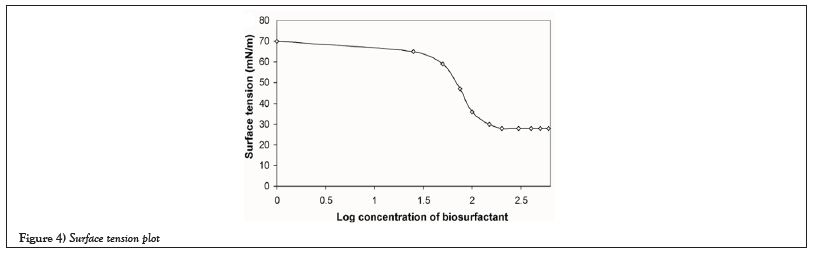
Figure 4: Surface tension plot.
With respect to the optimization of oil concentration, different concentrations of oil were inoculated in 50 ml of broth with 20 µl of cell concentration were given and incubated individually for 48 hours. Maximum percentage of removal/solubilization of crude oil was observed in 1 ml aqueous medium (Figure 5).
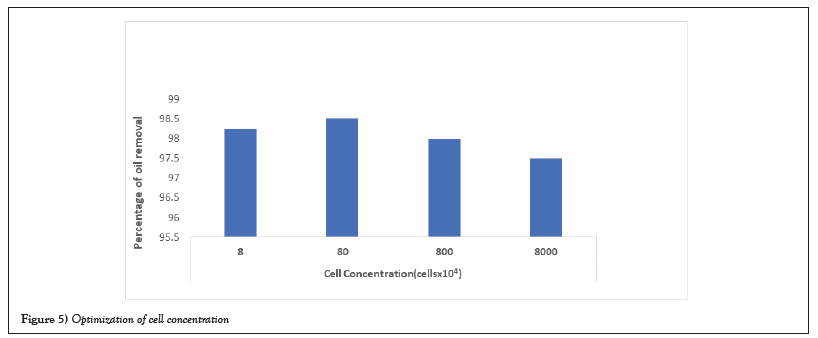
Figure 5: Optimization of cell concentration.
After the incubation period of 48 hours, the crude oil was solubilized and transformed into thread like structures.
Figure 6 illustrated the removal of crude oil contaminated soil within 48 hours of incubation. Similar to the aqueous medium, removal of crude oil in contaminated soil is above 90% within 48 hours of incubation.
With respect to comparison of surfactants at different concentration and time interval under shaking condition, higher incubation, concentration gives appreciable removal oil. Bio surfactants are equally good when comparing to the synthetic surfactant under shaking conditions. More than 80% of crude oil was removed with Phospholipid, SDS and Tween. More than 70% of crude oil was removed withTritonx-100 and CTAB. Bio surfactant obtained from the marine microbial source removed more than 90% of the crude oil (Figure 6).
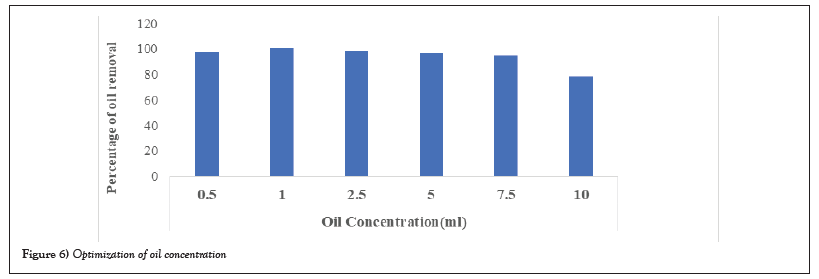
Figure 6: Optimization of oil concentration.
With respect to static condition, more than 90% of the crude oil which was mixed with biosurfactant, phospholipid, SDS and triton × 100 was removed. Less than 30% removal was observed with tween and CTAB. At higher concentration and higher incubation time tween 80 can remove more than 80% of the crude oil but CTAB was able to remove only less than 50%.
With respect to the column technique, there has been no removal of surfactant when the concentration is around 1% and at higher concentration the hexane extract became colloid, the reason why it is difficult to estimate the removal of crude oil. At 5% appreciable removal of biosurfactant was observed when compared to others. In beach soil, 56, 59 and 68% of crude oil was removed with Tween-80, SDS and biosurfactant respectively. In sandy soil, more than 55% of oil was removed with Triton, SDS and biosurfactant. No much removal in clay and clay loamy soil. In clay and clay loamy soil, more than 30% of oil was removed with tritonx-100 and biosurfactant from marine source. Figure 7 illustrated that biosurfactant treated and untreated soil samples. When surfactants present in a dynamic equilibrium between the continuous phase (water) and the disperse (oil) phase at interface, the surfactants with relatively high affinities towards aqueous phase can desorb from a droplet and diffuse through the aqueous phase, especially in marine oil spill scenarios when water is typically more abundant [22,23]. This may leave the droplet interfaces relatively vulnerable for coalescence [24]. Therefore, surfactants with higher affinity towards the oil phase may have a slight upper-hand in this regard, such as lechitin (Figure 7) [22].
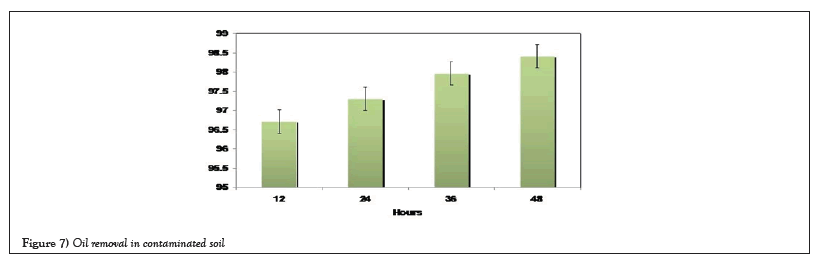
Figure 7: Oil removal in contaminated soil.
Instrumental analysis of crude oil and bio transformed sample
Spectral analysis UV-visible: The electrostatic charge plays a primary role in sustaining cell activities and behaviors through influencing the overall cell polarity and maintaining the degree of surface hydrophilicity [25]. The electrical potential of the interfacial region between the bacterial surface and the aqueous environment has been widely used to assess the net cell surface charge [25]. Dissipation of potential of Bacillus sp biomasses were assessed and the results are presented in Figure 8 illustrated that the crude oil and crude oil treated with bio surfactant sample which is extracted with hexane and subjected to UV-Visible spectrum. UV-Visible spectrum analyses showed presence of protein compounds (a small humpin the region of 280-290 nm). There is change in the crude oil peaks and some peaks also shifted (Figure 8).
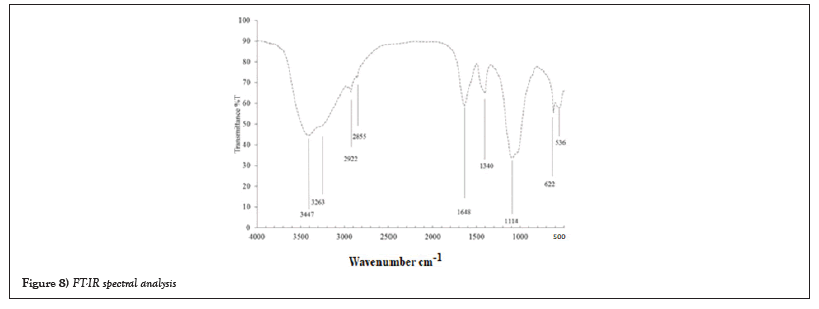
Figure 8: FT-IR spectral analysis.
FT-IR spectral analysis
They are lately hydrated and subsequently convert to the forms of carbonates and phosphates [26]. The alkane C-H bond stretch (2700-3000 cm-1) and carboxylic and/or hydroxyl groups (3200-3600 cm-1) were identified from the FTIR analysis. The presence of Al and Fe as oxygen functional groups within Al-OH and Fe-OH (800-900 cm-1) can be confirmed from Putra et al., [27]. The FTIR spectrum of samples after the incubation process was also presented in FT-IR analysis of the crude oil showed peaks at 3000-2850, 3000-2850, 2250-2100, 2250-2100, 1680-1600, 1450-1465, 1400-1000, 1400-1000, 1350-1000, 1000-650 cm-1 corresponding to C–H, C–H, C≡C, C≡C, C=C,-CH3,-CH2, CH, CH, C–N, =C-H- respectively. Figure 8 illustrated that the hexane extracted sample from the crude oil contaminated soil showed peaks at 3650-3600, 3500-3100, 2270-1940, 1680-1630, 1690-1640, 1680-1600, 1400-1000, 1350-1000, 1350-1140, 785-540 cm-1 corresponding to O-H, N-H, =C=O, C=O, C=N, C=O, C-H, N-H, S=O, C-H respectively [28].
Conclusion
This study helped to explain the biologically derived surface-active agents on removal of oil from oil spilled soil. In this study, compared with using crude oil removal rate was significantly increased using biosurfactant based washing solution. The mechanism of biosurfactant enhanced crude oil removal is closely related with its concentration. When the concentration of biosurfactant solution was below its Critical Micelle Concentration (CMC) value, the mechanism of biosurfactant enhanced crude oil removal. The lowered interfacial tension thus led to an increased contact angle and reduced capillary force holding the crude oil and soil particles and consequently enhanced the mobility of crude oil. When the concentration of biosurfactant is above its CMC value, the formation of biosurfactant micelle can greatly increase the solubilization process, and help to solubilize the residue oil compounds left in the soil system and enhance the removal of organic contaminants.
Acknowledgement
The authors extend their sincere thanks to The Chairman, Marudupandiyar College, Thanjavur for providing the facilities and The Bharathidasan University, Tiruchirappalli, Tamilnadu, India, recognizing our research work and that successful completion of the work.
References
- Yang C, Kaipa U, Mather QZ, et al. Fluorous metal–organic frameworks with superior adsorption and hydrophobic properties toward oil spill cleanup and hydrocarbon storage. J Am Chem Soc. 2011; 133(45):18094-18097.
[Crossref] [Google Scholar] [PubMed]
- Kingston PF. Long-term environmental impact of oil spills. Spill Sci Technol Bull. 2002; 7(1-2):53-61.
- Chen J, Zhang W, Wan Z, et al. Oil spills from global tankers: Status review and future governance. J Clean Prod. 2019; 227:20-32.
- Statham PJ. Nutrients in estuaries—An overview and the potential impacts of climate change. Sci Total Environ. 2012; 434:213-227.
[Crossref] [Google Scholar] [PubMed]
- Sheppard CR. Seas at the millennium: an environmental evaluation: 1. Regional chapters: Europe, The Americas and West Africa. 2000.
- Beyer J, Trannum HC, Bakke T, et al. Environmental effects of the Deep water Horizon oil spill: a review. Mar Pollut Bull. 2016; 110(1):28-51.
[Crossref] [Google Scholar] [PubMed]
- Trustees DN. Deep water Horizon oil spill: final programmatic damage assessment and restoration plan and final programmatic environmental impact statement. Deep water Horizon. 2016.
- Mignucci-Giannoni AA. Assessment and rehabilitation of wildlife affected by an oil spill in Puerto Rico. Environ Pollut. 1999; 104(2):323-333.
- Saravanan P, Prabagaran SR, Venkata Nancharaiah Y, et al. Isolation and characterization of Pseudoalteromonas ruthenica (SBT033), an EPS-producing biofilm bacterium from the seawater intake point of a tropical power station: EPS producing Pseudoalteromonas ruthenica. World J Microbiol Biotechnol. 2008; 24:509-515.
- Tugrul T, Cansunar E. Detecting surfactant-producing microorganisms by the drop-collapse test. World J Microbiol Biotechnol. 2005; 21:851-853.
- Maneerat S, Phetrong K. Isolation of biosurfactant-producing marine bacteria and characteristics of selected biosurfactant. Songklanakarin J Sci Technol. 2007; 29(3):781-791.
- Yakimov MM, Golyshin PN, Lang S, et al. Alcanivorax borkumensis gen. nov., sp. nov., a new, hydrocarbon-degrading and surfactant-producing marine bacterium. Int J Syst Bacteriol. 1998; 48(2):339-348.
[Crossref] [Google Scholar] [PubMed]
- Lai Q, Wang J, Gu L, et al. Alcanivorax marinus sp. nov., isolated from deep-sea water. Int J Syst Evol Microbiol. 2013; 63:4428-4432.
[Crossref] [Google Scholar] [PubMed]
- Pruthi V, Cameotra SS. Effect of nutrients on optimal production of biosurfactants by Pseudomonas putida—a Gujarat oil field isolate. J Surfactants Deterg. 2003; 6(1):65-68.
- Lotfabad TB, Shourian M, Roostaazad R, et al. An efficient biosurfactant-producing bacterium Pseudomonas aeruginosa MR01, isolated from oil excavation areas in south of Iran. Colloids Surf B. 2009; 69(2):183-193.
[Crossref] [Google Scholar] [PubMed]
- Yakimov MM, Timmis KN, Wray V, et al. Characterization of a new lipopeptide surfactant produced by thermotolerant and halotolerant subsurface Bacillus licheniformis BAS50. Appl Environ Microbiol. 1995; 61(5):1706-1713.
[Crossref] [Google Scholar] [PubMed]
- She YH, Zhang F, Xia JJ, et al. Investigation of biosurfactant-producing indigenous microorganisms that enhance residue oil recovery in an oil reservoir after polymer flooding. Appl Biochem Biotechnol. 2011; 163:223-234.
[Crossref] [Google Scholar] [PubMed]
- Gudiña EJ, Pereira JF, Rodrigues LR, et al. Isolation and study of microorganisms from oil samples for application in microbial enhanced oil recovery. Int Biodeterior Biodegradation. 2012; 68:56-64.
- Wang J, Ji G, Tian J, et al. Functional characterization of a bio surfactant-producing thermo-tolerant bacteria isolated from an oil reservoir. Pet Sci. 2011; 8:353-356.
- Rosen MJ, Kunjappu JT. Surfactants and interfacial phenomena. John Wiley & Sons; 2012.
- De Almeida DG, Soares Da Silva RD, Luna JM, et al. Biosurfactants: promising molecules for petroleum biotechnology advances. Front Microbiol. 2016; 7:1718.
[Crossref] [Google Scholar] [PubMed]
- Athas JC, Jun K, McCafferty C, et al. An effective dispersant for oil spills based on food-grade amphiphiles. Langmuir. 2014; 30(31):9285-9294.
[Crossref] [Google Scholar] [PubMed]
- Riehm DA, McCormick AV. The role of dispersants’ dynamic interfacial tension in effective crude oil spill dispersion. Mar Pollut Bull. 2014; 84(1-2):155-163.
[Crossref] [Google Scholar] [PubMed]
- Morrison ID, Ross S. Colloidal dispersions: suspensions, emulsions, and foams. New York: Wiley-Interscience; 2002.
- Wilson WW, Wade MM, Holman SC, et al. Status of methods for assessing bacterial cell surface charge properties based on zeta potential measurements. J Microbiol Methods. 2001; 43(3):153-164.
[Crossref] [Google Scholar] [PubMed]
- Demeyer A, Nkana JV, Verloo MG. Characteristics of wood ash and influence on soil properties and nutrient uptake: an overview. Bioresour Technol. 2001; 77(3):287-295.
[Crossref] [Google Scholar] [PubMed]
- Koswojo R, Utomo RP, Ju YH, et al. Acid Green 25 removal from wastewater by organo-bentonite from Pacitan. Appl Clay Sci. 2010; 48(1-2):81-86.
- Panjiar N, Sachan SG, Sachan A. Biosurfactants: a multifunctional microbial metabolite. Microbial Applications: Biomedicine, Agriculture and Industry. 2017:213-229.
Author Info
Department of PG and Research of Microbiology, Maruthupandiyar College, Affiliated to Bharathidasan University, Trichy, Pillaiyarppatti, Vallam, Tamil Nadu, IndiaReceived: 18-Mar-2023, Manuscript No. AGBIR-23-92249; Editor assigned: 22-Mar-2023, Pre QC No. AGBIR-23-92249 (PQ); Reviewed: 05-Apr-2023, QC No. AGBIR-23-92249; Revised: 12-Apr-2023, Manuscript No. AGBIR-23-92249 (R); Published: 01-May-2023, DOI: 10.35248/0970-1907.23.39.531-536
Citation: Suganthi B, Bharathidasan R. Comparative studies on role of synthetic and biologically derived surface-active agents on removal of oil from oil spilled soil. AGBIR.2023; 39(3):531-536.
Copyright: This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http:// creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com