Genistein: A new Isoflavone from Iraqi Chrozophora tinctoria (Euphorbiaceae): Its Extraction, Isolation and Structure Elucidation
Abstract
In the current study, genistein proof of existence as a novel metabolite in the Iraqi Chrozophora tinctoria (Euphorbiaceae) was the mainstay issue. Extraction of genistein was performed from the dried aerial parts of the Iraqi Chrozophora tinctoria by using soxhelt apparatus with 85% Methanol followed by fractionation with petroleum ether and ethyl acetate fraction. Preparative Reverse-phase high performance liquid chromatography HPLC analysis of the ethyl acetate fraction for the aerial parts of the plant yielded eight phytoconstituents one of them (peak NO.2) gave identical match with genistein standard, depending on which the isolation and quantitative determination were done. A fruitful product of amorphous powder was yielded with a concentration 5.7 μg/g, the structure of this compound was elucidated unambiguously by melting point, analytical RP-HPLC, Ultra Violet UV spectroscopic, Fourier Transform Infrared Spectroscopy FTIR spectroscopic and High-performance compact mass spectrometer (CMS) techniques revealing identical similarity to genistein.
Keywords
Chrozophora tinctoria, Genistein, RP-HPLC, UV spectroscopic and High-performance compact mass spectrometer (CMS)
Introduction
Chrozophora tinctoria (Figure 1) is a medicinal plant that belongs to the Euphorbiaceae family and contains approximately nine species, the majority of which are monoecism herbs [1]. Species from this genus have been found in the Mediterranean, tropical Africa, and Southwest Asia [1]. The plant was traditionally used to treat warts, an emetic, cathartic, and fever treatment [2]. In some studies, Chrozophora was used as an antioxidant [1], antimicrobial [2,3], anticancer [4], and wound healing in diabetics [5]. The Chrozophora genus is primarily composed of diterpenoids, alkaloids, coumarins, chromones, xanthones, sterols, phenolic compounds, and flavonoids [1,3,6,7]. This plant is high in flavonoids such as apigenin, rutin, quercetin, and acacetin [6,8-10].
Figure 1. Photo of Iraqi Chrozophora tinctoria
Isoflavone is a flavonoid (natural phenolic compound) with estrogen-like properties (phytoestrogen) [11,12]. Several isoflavones have been identified as cancer preventative agents as well as inducers of cell death via caspase-independent pathways in drug-resistant cancer cell lines [13]. Other therapeutic activities in cardiovascular disease, diabetes, and postmenopausal disorders have been discovered [14]. Genistein [4`,5,7-trihydroxyisoflavone or 5,7-dihydroxy-3-(4- hydroxyphenyl) chromen-4-one] (C15H10O5) belongs to the isoflavonoid family and has a 15-carbon skeleton. Genistein (Figure 2) is a typical phytoestrogenic compound with multiple biological activities [15]. It is a powerful antioxidant and ant browning agent in vitro and in vivo [16], and it is used for the prevention and treatment of many types of cancer. It has tumor-related enzyme-inhibitory properties, and it significantly inhibits skin carcinogenesis and cutaneous aging in mice and photo-damage in humans [16]. It is also used to treat postmenopausal syndrome [17], osteoporosis disease [18], and cardiovascular diseases in both animals and humans [19].
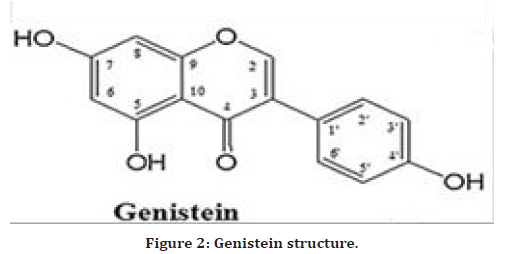
Figure 2. Genistein structure
Aim of study
The hypothesis of the study concerned about other type of flavonoid in the Iraqi Chrozophora tinctoria aerial parts (isoflavone) which has different pharmacological activities applied in alternative medicine with wide future applications, leading the way by extraction, isolation and structure elucidation of the genistein from the Iraqi wild Chrozophora tinctoria.
Materials and Methods
Equipment and chemical
The instruments used were rotary evaporator (BȔCHI Rotavapor R-205, Swiss), sonicator (Baranson Sonifier, USA), high-performance liquid chromatography [HPLC (Knauer Germany 10AV-LC)], Fourier Transform Infrared Spectroscopy [FTIR (Schimadzu/Japan 1900)], High-performance compact mass spectrometer [CMS (ADVION/USA)]. All chemicals and solvents used were of analytical grade and obtained from Riedel-de Haen, Germany, except trifluoroacetic acid, and methanol which are HPLC grade purchased from Sigma-Aldrich, Germany. The standard genistein were purchased from Chengdu Biopurify Phytochemicals, China (purity >97).
Collection of plant materials and authentication
The plant materials of Chrozophora tinctoria (aerial parts) was collected during September–November 2020 from local fields about 2 Km northwest of Tikrit city. The plant was identified, confirmed and authenticated by Dr. Mohammed Amer taxonomist in biology department, college of science/ university of Tikrit. The plant was cleaned, dried in shade at room temperature, pulverized by electrical milled and then weighed.
Extraction of plant materials
A fifty grams of powdered plant material of Chrozophora tinctoria was packed in a thimble of soxhlet apparatus and extracted with 85% methanol until complete exhaustion (750ml for 12hrs). The methanolic extract was filtered and then the solvent was evaporated under reduced pressure using rotary evaporator at a temperature not exceeding 40 0C to give a dark reddish brown residue designated as a crude fraction [6,20]. The crude fraction was partitioned with Petroleum ether and Ethyl acetate (3x200 ml) for each fraction. The ethyl acetate fraction was dried over anhydrous sodium sulfate, filtered, evaporated to dryness, weighted and subjected for identification and purification procedures [7]. The optimized process for the extraction, isolation and identification of Genistein product from the dried powdered plant material (aerial parts) of Chrozophora tinctoria is shown in following scheme Figure 3.
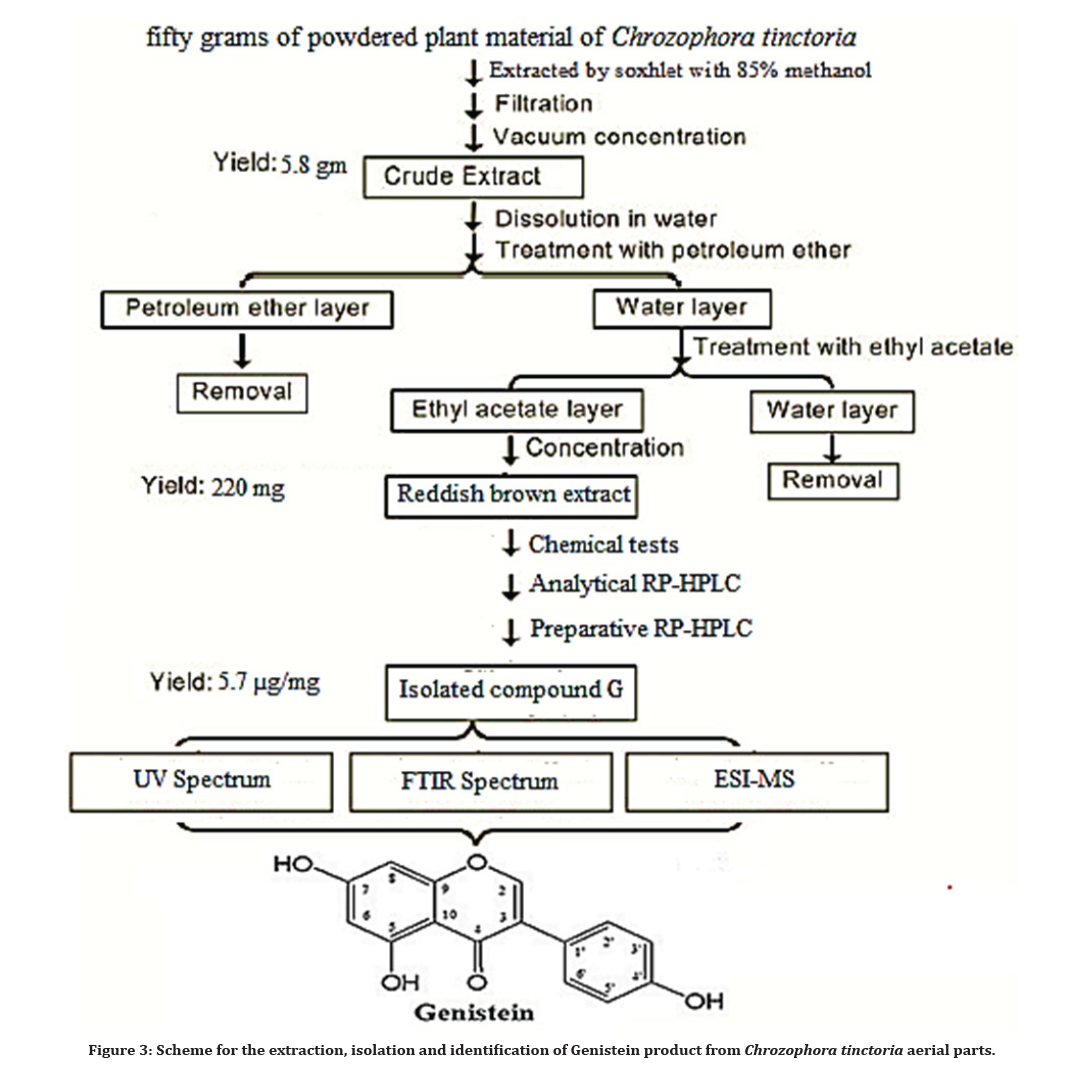
Figure 3. Scheme for the extraction, isolation and identification of Genistein product from Chrozophora tinctoria aerial parts.
Preliminary phytochemical investigation
Phytochemical profiling of crude methanolic extract and ethyl acetate fraction of Chrozophora tinctoria aerial parts were carried out using the procedures as described by Harborne et al. [21] and Lallianrawna et al [22].
Alkaline reagent test
Few milligrams of the crude extract and ethyl acetate fraction were suspended in ethanol and few drops of 5% ethanolic KOH were added, and then, few drops of 5% HCl were added. The changes in colors were recorded.
Shinoda test
1ml from extracts (crude and ethyl acetate) of Chrozophora tinctoria aerial parts treated with magnesium turning and concentrated HCl after few minutes’ reduction reaction gives orange-red color indicates the presence of flavonoids.
Reverse - phase high performance liquid chromatography analysis (RP-HPLC)
A fast, simple and sensitive RP-HPLC technique can provide a lot of information about the contents of the plant extract. It is also used for qualitative and quantitative determination of extract constituents by making a comparison of their retention time and the shape of the UV spectrum with calibration (linear least square regression equation) of the detected compounds with that for authenticated reference standards at identical chromatographic conditions. In this study, the ethyl acetate fraction for Chrozophora tinctoria aerial parts was analyzed by this technique.
Preparations of standard and samples for analysis
Standard solutions for HPLC of Genistein were prepared by dissolving 0.04 mg in 1 ml of methanol HPLC grade. Dried sample (ethyl acetate fraction) was prepared for HPLC analysis by dissolving it in methanol and subjecting it to ultrasonication at 60% duty cycles for 25 minutes at 25°C followed by centrifugation at 7500 rpm for 15 minutes. The clear supernatant of sample was evaporated under vacuum. The residues were suspended individually, in 1 ml of methanol HPLC grade, homogenizing using vortex mixer, and passing them through 2.5 μm disposable filter, and stored at 4°C for further analysis. 20 μl of the sample was injected into HPLC system for analysis [20].
RP-HPLC conditions for identification and qualitative analysis
Mobile phase: Gradient: Solvent A (1% acetic acid in HPLC grade water) and solvent B (acetonitrile) (0-5min A:90% B:10%,5-20min A:60% B:40%,20-40 min A: 40% B:60%,40-50min A:10% B:90%,50-55min A:90% B:10%).
Column: Knauer/Germany LC C18 (250 mm x 4.6 mm, 5 μm particle size).
Sample: Ethyl acetate fraction for Chrozophora tinctoria aerial parts
Standard: Genistein.
Flow rate: 0.7 ml/min.
Injection volume: 50 μL.
Injection concentration: 1 mg/ml.
Detection: UV Detector at λ 280 nm [23].
RP-HPLC conditions for isolation and quantitative analysis
Mobile phase: gradient: solvent A (0.05% TFA in HPLC grade water) and solvent B (acetonitrile) (0-5min A:90% B:10%,5-20min A:70% B:30%,20-40min A:50% B:50%,40-50min A:30% B:70%,50-55min A:10% B:90%).
Column: Nucleodur, Macherey –Angel/Germany LC C18 (250 mm x 10 mm, 5 μm particle size).
Sample: ethyl acetate fraction for Chrozophora tinctoria aerial parts
Standard: Genistein.
Flow rate: 3 ml / min.
Injection volume: 100 μL.
Injection concentration: 1 mg /ml.
Detection: UV Detector at λ 220 nm [23].
Characterization and structure elucidation of the isolated constituent (G)
Isolated substance G was identified by different identification ways including:
Measuring the melting point and compare it to that of the standard.
9 Analytical HPLC that revealed the peak area at the same retention time with the standard
9 Analyzing the UV/Vis spectra and IR spectra to detect the higher absorbance and the active chemical groupings respectively
9 Analyzing the ESI-MS spectra to discover the molecular mass information.
Results and Discussions
In spite of the importance of Chrozophora tinctoria as Iraqi wild medicinal plants, the phytochemical studies concentrated on the flavonoids (secondary metabolites) especially flavone and flavonol glycoside types. Therefore; the chosen isoflavone compounds, especially genistein in this study is the main target for its different pharmacological activities and to do the first Iraqi study and perhaps worldwide about the present of genistein in the Iraqi Chrozophora tinctoria. The aerial parts were extracted by Soxhlet method, as this method is heat dependent which help solvent penetration through walls of the aerial part fragments, solvent type was based on literature survey; showed a majority of polar to moderately polar phytochemicals that exists in the Chrozophora tinctoria, hence; 85% methanol was used in this study in an attempt to obtain as much extract yield as possible from the plant. From a scientific point of view, differences in polarities is used as a crucial separation step in order to analyse interconnected chemicals in extracts preceded by different polarity petroleum ether and ethyl acetate fractionations solvents and the weight yield of each fraction is illustrated in the Table 1..
| Fractions name | Weight Yields |
|---|---|
| Crude methanolic extract | 5.8 gm |
| Petroleum ether | 670 mg |
| Ethyl acetate | 220 mg |
Table 1: Difference in weight yields of extractâ??s content of Iraqi Chrozophora tinctoria aerial parts
Preliminary phytochemical investigation
The results of the phytochemical screening confirm the presence of flavonoids in both crud methanolic extract and ethyl acetate fraction. The results gained resembles other studies (researches and reviews) on same aspect previously done [24,25].
Reverse - phase high performance liquid chromatography analysis (RP-HPLC)
The chromatogram results reveals that, the RP- HPLC method was efficient for qualitative screening and quantitative determination of new isoflavone (genistein) in the Iraqi Chrozophora tinctoria aerial parts ethyl acetate fraction, in which both genistein standard and one compound (peak NO.2) of the eight phytoconstituents in ethyl acetate fraction have similar retention times (7.2 minutes) as shown in Figure 4.
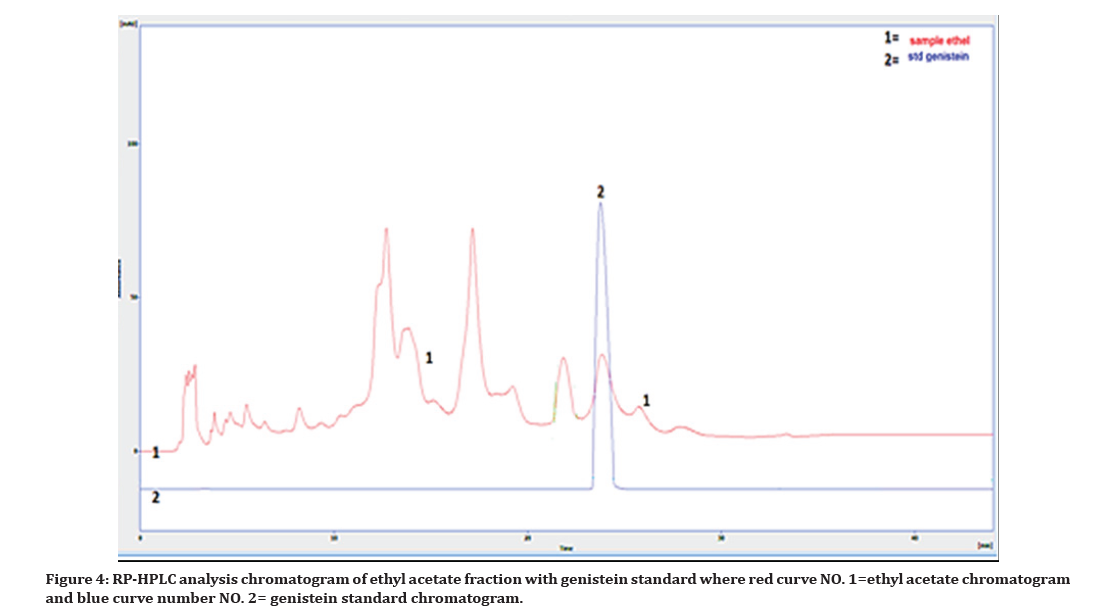
Figure 4. RP-HPLC analysis chromatogram of ethyl acetate fraction with genistein standard where red curve NO. 1=ethyl acetate chromatogram and blue curve number NO. 2= genistein standard chromatogram.
According to linear least square regression equation, quantitative determination was carried out by using a calibration curve for the isolated compound G with a correlation factor equals’ 0.9996687 and peak areas of the new detected compound in fraction of ethyl acetate was used to determine the concentration as shown in Table 2 and Figure 5.
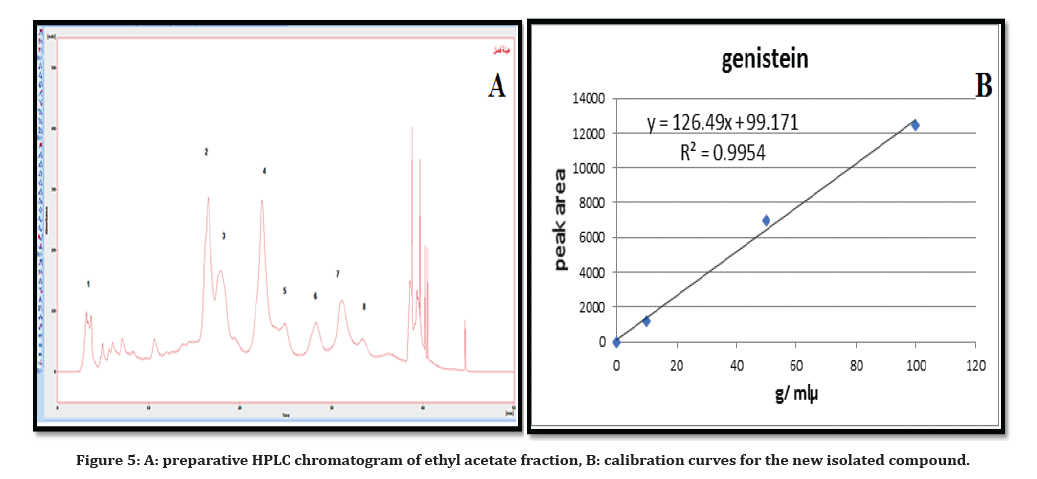
Figure 5. A: preparative HPLC chromatogram of ethyl acetate fraction, B: calibration curves for the new isolated compound
| Sample | Peak area | Concentration in ethyl acetate fraction µg/ml | Concentration in plant µg/mg |
|---|---|---|---|
| Ethyl acetate | 710.106 | 6.1779222 | 5.702697415 |
Table 2: The concentration of the isolated compound G from Iraqi Chrozophora tinctoria aerial parts ethyl acetate fraction
Identification, characterization and structural elucidation of isolated G
The characterization and structural elucidation of the amorphous powder isolated G constituent was done by using the following techniques:
Melting point
The melting point is a physical property which gives useful information, which in its turn, helps to identify the sample and its purity. The isolated G constituent had a melting point at 300 C0- 302C0 (with decomposition) compared with standard genistein which has a melting point of 301C0-302C0 (with decomposition).
Analytical RP-HPLC
For further identification, a qualitative HPLC analysis was applied on the isolated G constituent, in which the identification was occurred by comparison of retention times obtained at identical chromatographic conditions of the isolated G constituent and the authentic standard (genistein), as shown in Figure (6A), the HPLC chromatograms were identical for both the isolated compound G and genistein standard.
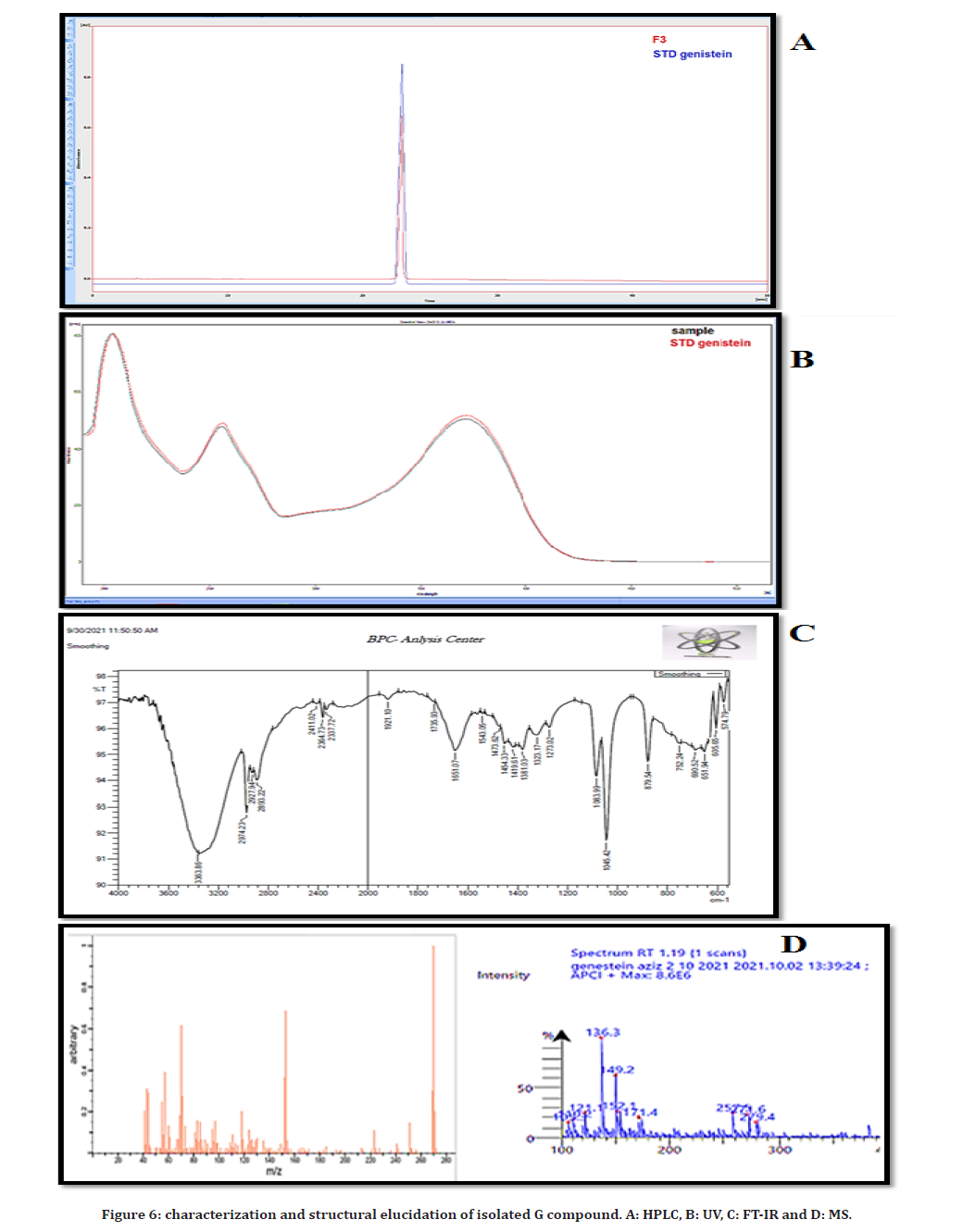
Figure 6. characterization and structural elucidation of isolated G compound. A: HPLC, B: UV, C: FT-IR and D: MS.
UV/Vis spectra
Determination of UV spectrum analysis was done by UV computerized spectrophotometer (SHIMADUZU-1900/ range 200-1000 nm) using 100% methanol as standard reference. The isolated G constituent showed identical spectrum and same lambda max with genistein standard at same condition, as shown in Figure (6B).
Fourier Transform Infrared Spectroscopy (FTIR)
FTIR with attenuated total reflection (ATR) technique/ model: SHIMADUZU1900/range 550-4000 nm was used to detect the main active chemical groups of the isolated G constituent: the peak at 3363.86cm−1, a broad band is most probably the result of O-H stretching vibrations of phenol OH group. The peak at 2947.23 cm−1 showed C-H stretching due to –CH3. The peak at 1651.07cm−1 indicates the presence of -C=O, carbonyl group. The peak at 1606.76 cm−1 showed the presence of -CH=CH group. The peak at 1508.38 cm−1 indicates the presence of benzene ring. The peak at 860.28 cm−1 showed the presence of substitution of benzene ring of isolated compound. FTIR spectrum was proved that the isolate G constituent has identical peaks at same region to genistein standard as shown in Figure (6C).
High-performance compact mass spectrometer (CMS)
The sample is typically dissolved in a solvent usually methanol and pumped through a capillary inside an uncharged quartz tube. The temperature was: 300 °C, the run time: 3 min with Gas flow rate: 4L/min. Mass spectra fill scan range was: 20-1020 m/z and Injection volume: 1μL. Mass spectral data shows molecular ion peak ��/��=271.5 which has a moderate abundance with other mass peaks (105,110,121,136,149,152,171,257) which were identical with the peaks of genistein standard, as shown in Figure (6D). Based on the above spectral and chemical studies data giving suggestion that compound G is having an isoflavonoid skeleton with two phenolic hydroxyl groups, all of these lead to the conclusions confirming that the isolated G compound was genistein.
Conclusion
Diet is predicted to contribute to about 50% of cancers and other major chronic diseases in the advanced world population, while incorporating food rich with phytoestrogen in human diet for healthcare and various traditional medicinal systems around the world, had been proved to lower the risk of development of several devastating diseases like cardiovascular, neurological and other degenerative disorders as do for cancers. In this study, the Iraqi Chrozophora tinctoria aerial parts were discovered as a novel natural source for genistein with a concentration of 5.7 μg/g, being the first Iraqi study and perhaps worldwide about the presence of genistein in the Iraqi Chrozophora tinctoria. A simple and easy method of extraction accompanied with rapid and sensitive RP-HPLC technique is successfully developed. The high throughput, simple and sensitive techniques facilitate the accurate quantification of genistein isoflavone in complex matrices such as plant extracts without primary purification in a short duration.
References
- Marzouk MM, Hussein SR, Kassem ME, et al. Phytochemical constituents and chemosystematic significance of Chrozophora tinctoria (L.) Raf. Nat Prod Res 2016; 30:1537-1541.
- Dastagir G, Hussain F, Khan AA. Antibacterial activity of some selected plants of family Zygophyllaceae and Euphorbiaceae. J Med plants Res 2012; 6:5360â??5368.
- Usman H, Musa YM, Ahmadu AA, et al. Phytochemical and antimicrobial effects of Chrozophora senegalensis. African J Tradit Complement Altern Med 2007; 4:488â??494.
- Abdel-Naim AB, Alghamdi AA, Algandaby MM, et al. Rutin isolated from Chrozophora tinctoria enhances bone cell proliferation and ossification markers. Oxid Med Cell Longev 2018; 2018.
- Maurya H, Semwal M, Dubey SK. Pharmacological evaluation of Chrozophora tinctoria as wound healing potential in diabetic rat’s model. Biomed Res Int 2016; 2016.
- Hawas UW. Antioxidant activity of brocchlin carboxylic acid and its methyl ester from Chrozophora brocchiana. Nat Prod Res 2007; 21:632â??640.
- Hussein IH, Mirghani MES, Man YBC. Physicochemical characteristics of Argessi (Chrozophora brocchiana), Kenaf (Hibiscus cannabinus) and Loofah (Luffa cylindrica) seed oils. Sudan J Agric Res 2006; 6:53â??60.
- Gibbs RD. Chemotaxonomy of flowering plants: Four volumes. McGill-Queen’s Press-MQUP 1974.
- Abdallah HM, Almowallad FM, Esmat A, et al. Anti-inflammatory activity of flavonoids from Chrozophora tinctoria. Phytochem Lett 2015; 13:74â??80.
- Hecker E. Cocarcinogenic principles from the seed oil of Croton tiglium and from other Euphorbiaceae. Cancer Res 1968; 28:2338â??2348.
- Manach C, Scalbert A, Morand C, et al. Polyphenols: Food sources and bioavailability. Am J Clin Nutr 2004; 79:727â??747.
- Ogbuewu IP, Uchegbu MC, Emenalom OO, etal. Overview of the chemistry of soy isoflavones, potential threats and potential therapeutic benefits. Electron J Environ Agric Food Chem 2010; 9.
- Sarkar FH, Li Y. Soy isoflavones and cancer prevention: Clinical science review. Cancer Invest 2003; 21:744â??57.
- Prasad S, Phromnoi K, Yadav VR, et al. Targeting inflammatory pathways by flavonoids for prevention and treatment of cancer. Planta Med 2010; 76:1044â??1063.
- Spagnuolo C, Russo GL, Orhan IE, et al. Genistein and cancer: current status, challenges, and future directions. Adv Nutr 2015; 6:408â??19.
- Mazumder MAR, Hongsprabhas P. Genistein as antioxidant and antibrowning agents in in vivo and in vitro: A review. Biomed Pharmacother 2016; 82:379â??92.
- Evans M, Elliott JG, Sharma P, et al. The effect of synthetic genistein on menopause symptom management in healthy postmenopausal women: A multi-center, randomized, placebo-controlled study. Maturitas 2011; 68:189â??196.
- Yamaguchi M, Gao YH. Inhibitory effect of genistein on bone resorption in tissue culture. Biochem Pharmacol 1998; 55:71â??76.
- Altavilla D, Crisafulli A, Marini H, et al. Cardiovascular effects of the phytoestrogen genistein. Curr Med Chem Hematol Agents 2004; 2:179â??186.
- Abdul-Jalil TZ, Saour K, Nasser A-M. Phytochemical study of some flavonoids present in the fruits of two Ammi L. species wildly grown in Iraq. Iraqi J Pharm Sci 2010; 19:48â??57.
- Harborne AJ. Phytochemical methods a guide to modern techniques of plant analysis. Springer Science and Business Media 1998.
- Lallianrawna S, Muthukumaran R, Ralte V, et al. Determination of total phenolic content, total flavonoid content and total antioxidant capacity of Ageratina adenophora (Spreng.). King H Rob Sci Vis 2013; 13:149â??156.
- Seal T. Quantitative HPLC analysis of phenolic acids, flavonoids and ascorbic acid in four different solvent extracts of two wild edible leaves, sonchus arvensis and oenanthe linearis of north-eastern region in India. J Appl Pharm Sci 2016; 6:157â??166.
- Delazar A, Talischi B, Nazemiyeh H, et al. Chrozophorin: A new acylated flavone glucoside from Chrozophora tinctoria (Euphorbiaceae). Revista Br Farmacog 2006; 16:286-90.
- Al-Snafi AE. The chemical constituents and pharmacological importance of Chrozophora tinctoria. Int J of Pharm Rev Res 2015; 5:391-396.
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Author Info
Department of Pharmacognosy and Medicinal Plants, College of Pharmacy, University of Baghdad, IraqReceived: 07-Mar-2022, Manuscript No. JRMDS-22-52361; Editor assigned: 09-Mar-2022, Pre QC No. JRMDS-22-52361 (PQ); Reviewed: 23-Mar-2022, QC No. JRMDS-22-52361; Revised: 28-Mar-2022, Manuscript No. JRMDS-22-52361 (R); Published: 04-Apr-2022