A Comparative Study of Tissue-Cultured Iraqi Medicago sativa with their Ordinary Cultivated by Reverse-Phase High Performance Liquid Chromatography (RP-HPLC)
Abstract
Medicago sativa L. (alfalfa) is one of the most cultivated plants which have high nutritional value as fodder since antiquity and to treat many health disorders related to secondary metabolites with antioxidant and anti-inflammatory effects. For the first time, a comparative study was applied for bioactive constituents of tissue cultured and their ordinary counterparts of Iraqi Medicago sativa. Two types of agriculture techniques were employed, plant tissue cultured (three different tissue culture types) and ordinary agriculture (two different aerial parts and growth stages). The reverse-phase high-performance liquid chromatography applied for qualitative and quantitative of different phytochemicals in this plant giving an idea about the differences in the availabilities of these phytochemicals like steroids (Beta-sitosterol, Stigmasterol), phenolic acids (Caffeic acid, Chlorogenic acid, Gallic acid), and flavonoids (Myricetine, Naringinine) by the differentiated techniques.
Keywords
Medicago sativa L, Tissue culture, Reverse–phase high-performance liquid chromatography (RP-HPLC)
Introduction
The perennial plant Medicago sativa L., also known as alfalfa, belongs to the Fabaceae family. It has been cultivated since antiquity and is utilized in both cookery and phytotherapy. Over time, it has been regarded solely as a fodder plant, but it is now gaining popularity because of the minerals and bioactive chemicals it contains, which can have a favorable impact on health. Alfalfa can be regarded as a natural multivitamin because of its nutritional qualities and vitamin and mineral content. The plant's above-ground sections are high in beta carotene, vitamins C and E, as well as minerals like potassium, iron, calcium, magnesium, and silicon. Furthermore, alfalfa has a cleansing impact and helps to prevent intestinal malignancies due to its high chlorophyll content. The root of this plant contains triterpene saponins that decrease cholesterol without damaging the good fraction (HDL), protect against arteriosclerosis, and induce atherosclerosis regression. Furthermore, the European Food Safety Authority (EFSA) has approved this species as a safe dietary supplement [1]. Scientists are devoting increasing attention to Lucerne or Alfalfa (Medicago sativa L.) in the food sector recently since the demand for green food has increased. Lucerne is a drought-tolerant, perennial grass [2] a herbaceous legume forage [3]. Lucerne protein, which is derived from lucerne leaves, is a suitable source for generating healthy and functional foods [4]. However, alfalfa was mentioned in Babylonian texts in 700 BC [5]. Additionally, the Mesopotamian plain (Iraq) was a traditional meeting ground for ancient races from Asia, Africa, and Europe [6].
Implying it was cultivated by then. From its origins, Alfalfa spread across much of Europe, North Africa, the Middle East, and Central and Northern Asia. It was introduced into North and South America in the 16th century, and Australia in the 1800s [7]. The growth of cells, tissues, and organs in a specified solid or liquid medium is known as plant tissue culture. Plant tissue culture technologies are used to mass-produce plants on a large scale. In suspension cultures and bioreactors, axillary buds and somatic embryos are only employed in small amounts. Micropropagation includes steps such as prepropagation, explant initiation, explant proliferation culture, shooting and rooting, and hardening [8]. RPHPLC is a chromatographic process that can separate a mixture of substances and is used in phytochemical and analytical chemistry to identify, quantify, and purify the different components of the mixture [9]. This methodology is currently gaining traction as the recommended method for fingerprinting investigations for herbal plant quality control, according to various analytical techniques [10].
Material and Methods
In vivo and in vitro growth protocol, plant materials and extraction
In Vivo-plant material
Aerial parts (steam, leaves and flowers) of Alfalfa (Medicago sativa) which grow as cultivated plant in Iraq, were collected during the months of (June- November) for leaves and flowers from district AL_Hilla city farms located at Babylon-Iraq, then authenticated by the herbarium of the department of Horticulture and landscape\College of Agriculture\Al-Qasim Green University. These parts were left to dry in shade, then grinded using an electrical blender, weighted and then extracted.
In Vitro-Tissue culture materials
The type of tissue culture done in this study was seed culture and callus culture, at different culture media and different circumstance, the work done at Collage of agriculture\University of Al Kufa, then the explant of seed culture propagates at different type of light:
✓ White light, mimic the ordinary light.
✓ Red light, to make the explant propagate at tensive circumstances, to know the explant ability to make differences in concentration and availability of secondary metabolites in comparison to white light.
Callus culture, was done at different type of culture media and different circumstances in which the explant propagates at dark chambers, then explants of all types mentioned above collected individually, washed, dried and grinded to fine particles (Figure 1).

Figure 1. Iraqi Medicago sativa, ordinary plant and tissue cultured at different circumstances.
Study design
Comparison of different type, stages and parts of Iraqi Medicago sativa for ordinary plants and propagated seeds tissue culture explants, taking two different stages of growth for plant aerial parts (leaves and stems), while the flowers were collected later on, as illustrated in Table 1.
| Sample | Types | Abbreviation |
|---|---|---|
| Mature aerial plants | Leaves and stems | A |
| Un mature aerial plants | Leaves and stems | B |
| Flowers of mature plants | Flowers | C |
| Explant propagated under white light | Leaves and stems | D |
| Callus tissue culture | Callus mass | E |
| Explant propagated under red light | Leaves and stems | F |
Table 1: List of Iraqi Medicago sativa samples.
Extraction
Approximately 10gm of dried grounded aerial part of mature(45 day and more), 11gm of un-mature (25 day and less) and 12gm flowers alone (mature plant top flower) and approximately 2.9gm of explant tissue culture for normally propagated (under white light), 1gm of explant propagated under tensive light (red light) and 0.7gm of dried grounded callus type of this technique, were all subjected to extraction using Soxhlet apparatus with 85% ethanol (150ml) [11] then crude extracts was filtered, concentrated under reduced pressure using rotary evaporator and suspended in distilled water then partitioned successively with petroleum ether for defatting ( 100ml of petroleum ether) fraction 1 (F1).
After that the aqueous layer fraction concentrated under reduced pressure by the rotary evaporator to form (F2) fraction 2 [12,13].
The above step was done to make differentiated comparisons between different stages of the plant (mature and un-mature), different parts of the plant (aerial parts, flowers) and different applied types of tissue culture techniques for the evaluation of tissue culture on the concentrations of secondary metabolites (phytochemicals) selected to be identified here which were Beta-sitosterol and Stigmasterol as steroids, Caffiec acid, Chlorogenic acid and Gallic acid as phenolic acids, lastly Myricetin and Naringenine as flavonoids.
Identification of different bioactive constituents
Identification of different bioactive constituents was performed by:
Thin layer chromatography (TLC).
Reverse phase high performance liquid chromatography (RP-HPLC).
Qualitative identification by thin layer chromatography
An analytical TLC was performed for the six Iraqi Medicago sativa samples identification in which Silica gel TLC (GF 254) plate was used as a stationary phase, diffent developinging solvent system were applied and the best were Chloroform: ethyl acetate (80:20) and N-butanol: acetic acid: water (40:10:50), the six Iraqi Medicago sativa samples were applied on TLC plate. After development, detecting the chromatogram was done by UV spectrum 254nm and 365nm [14].
Qualitative and quantitative identification by reverse phase high performance liquid chromatography
Qualitative and quantitative determination of expected bioactive constituents in their six samples (A-F) obtained from different stages, parts and propagation of Iraqi Medicago sativa was carried out using reverse-phase high performance liquid chromatography (RP-HPLC). The identifications were made by comparison between retention times and UV/Vis spectrum matching obtained at identical chromatographic conditions of analyzed samples and authentic standards [15].
The following equation was used to calculate the percentage of the compound in the plant:
Percentage of compound in the plant=(AUC of plant sample/AUC of the standard) X Conc. St X DF X 100/ Weight of the dried plant used in the extraction
Where:
AUC=Area under the curve.
DF=Dilution factor.
Conc. St.=Concentration of the standard used in HPLC
HPLC conditions of petroleum ether fractions (F1)
Mobile phase: linear gradient: methanol (solvent A): acetonitrile (solvent B) (0-20min A:30% B:70%,20- 30min A:0% B:100%,30-45min A:30% B:70%)
Column: Knauer/Germany LC C18 (250 mm x 4.6 mm, 5 μm particle size).
Sample: petroleum ether fractions of six Iraqi Medicago sativa samples (A-F)
Standards: ß-sitosterol and stigmasterol
Flow rate: 0.7 ml / min
Injection volume: 50 μL
Injection concentration: 1 mg /ml
Detection: UV Detector at λ 210 nm [16].
HPLC conditions of aqueous layer fractions (F2)
Mobile phase: gradient: solvent A (1% acetic acid in HPLC grad water) and solvent B (acetonitrile) (0-5min A:90% B:10%,5-20min A:60% B:40%,20-40min A:40% B:60%,40-50min A:10% B:90%,50-55min A:90% B:10%).
Column: Knauer/Germany LC C18 (250 mm x 4.6 mm, 5 μm particle size).
Sample: aqueous layer fractions of six Iraqi Medicago sativa samples (A-F)
Standards: Caffiec acid, chlorogenic acid, myricetin, gallic acid and Naringenine
Flow rate: 0.7 ml / min
Injection volume: 50 μL
Injection concentration: 1 mg /ml
Detection: UV Detector at λ 272, 280 and 310 nm [17].
Results and Discussions
Different parts, stages and types of ordinary and tissue culture propagated Iraqi Medicago sativa extractions with different solvents were applied for the purpose to get the best extract with the highest concentration of the active constituents depend on the specific nature of these compounds.
Table 2 shows the fractions percentage of yield of extracts obtained from different parts of the plant, ccomparison between these percentages gave a good idea about extractions yield in which the flower of mature plant(C) gave the highest percent of extraction than the other areal parts of the plant, while tissue culture explant propagated at the Callus technique (E) gave the highest percent of extraction than the other tissue culture techniques.
| Fractions | A% | B% | C% | D% | E% | F% |
|---|---|---|---|---|---|---|
| Petroleum ether fractions | 3.28% | 1.65% | 0.58% | 3.79% | 9.57% | 4.60% |
| Aqueous layer fractions | 15.89% | 23.35% | 30.52% | 18.89% | 25.85% | 18.30% |
| Total % | 19.175 | 25% | 31.10% | 22.68% | 35.40% | 22.90% |
Table 2: Fraction extract percent of different parts, stages and type of ordinary and tissue culture propagated Iraqi Medicago sativa seeds.
The appliance of percent extract yielded comparison gave an idea about the preferred propagation technique, plant part and germination stages required to reach the best desired concentration of phytochemicals and open many gates for researchers to develop the desired one [18].
Qualitative identification of active constituents by thin layer chromatography (TLC)
Thin layer chromatography (TLC) is a primary beneficial analytical technique applied for the identification and qualification of different phytochemicals present in the targeted extract samples of different plant parts, germination stages and propagation technique [8].
TLC of different fractions obtained from Iraqi Medicago sativa plant by different developing systems are explained in Figure 2 and the following:
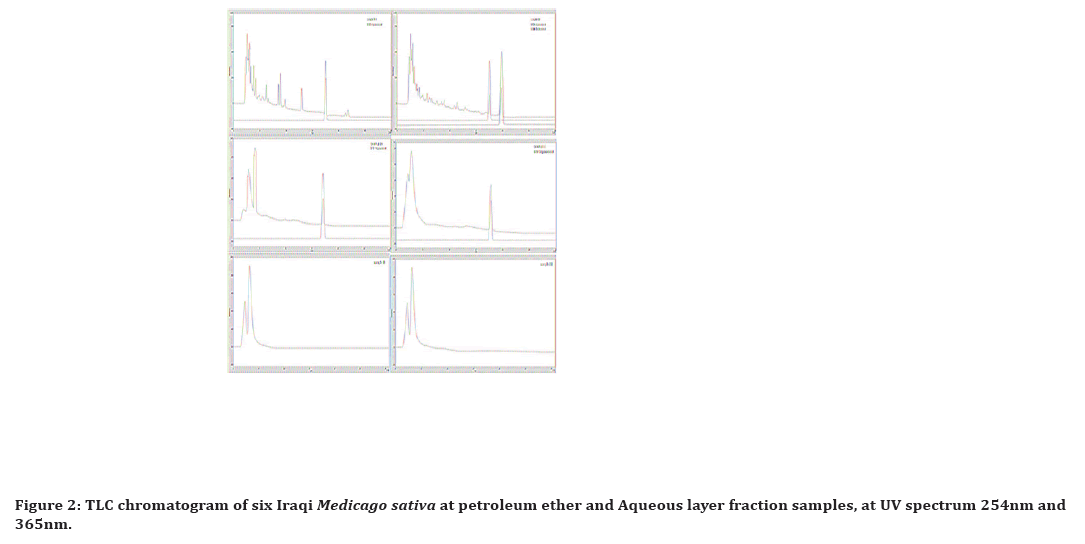
Figure 2. TLC chromatogram of six Iraqi Medicago sativa at petroleum ether and Aqueous layer fraction samples, at UV spectrum 254nm and 365nm.
Petroleum ether fractions 1:
The best yielding developing solvent system was Chloroform: ethyl acetate (80:20), in which the D1 fraction (plant tissue culture propagated at white light) gave more spots than others, while the E1 fraction callus tissue culture gave the least as illustrated in Figure 2.
Aqueous layer fractions 2
The best yielding developing solvent system was the N-butanol: acetic acid: water (40:10:50) in which the E2 fraction (callus tissue culture) gave more spots than others, while the A2 fraction (ordinary mature plant) gave the least as illustrated in Figure 2.
Fingerprinting of some phytochemicals by RP- HPLC analysis
Identification of some bioactive constituents by RPHPLC in ordinary plant (different stages and parts) and propagated tissue culture explants (two for seeds and one for callus) of Iraqi Medicago sativa as bellow.
RP-HPLC analysis for petroleum ether fraction
According to the RP-HPLC analysis of the six Iraqi Medicago sativa samples, Beta-sitosterol and stigmasterol were presented in sample A, while stigmasterol was present in all except sample E and F as apparent in Figure 3, showing the specific steroid compound profiles from different Iraqi Medicago sativa samples.
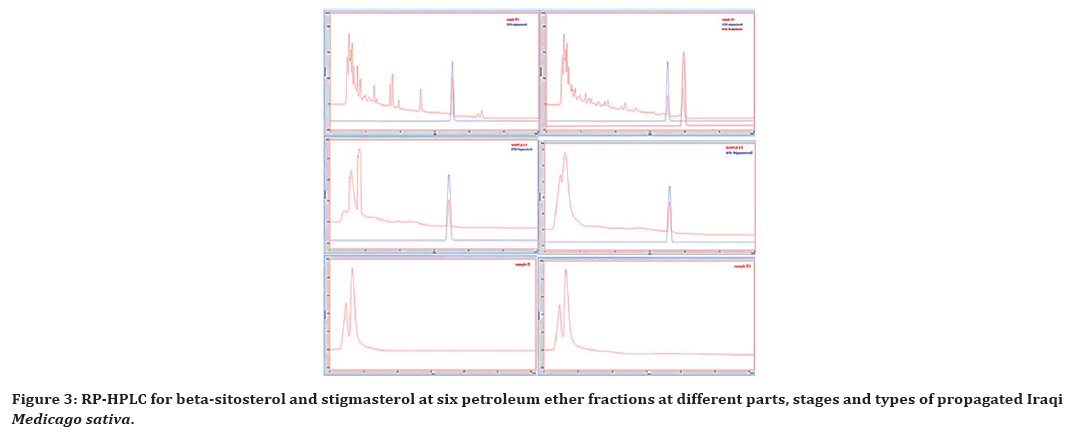
Figure 3. RP-HPLC for beta-sitosterol and stigmasterol at six petroleum ether fractions at different parts, stages and types of propagated Iraqi Medicago sativa.
Concerning quantitative determination of Betasitosterol and stigmasterol in the desired fractions serial dilutions of the standards were prepared. A plot of area vs concentration of Beta-sitosterol and stigmasterol standards shows a linear fit which was subsequently used for the quantification of Beta sitosterol and stigmasterol in the analyzed fractions as illustrated in Table 3.
| Phytochemical | HPLC results | |||
|---|---|---|---|---|
| Stigmasterol | SAmple | Stigmasterol peak area | STIGMASTEROL µg/ml | µg/gm dry powder of plant |
| A1 | 2032.957 | 30.1445421 | 1.581985569 | |
| B1 | 3124.523 | 46.8455019 | 0.308328576 | |
| C1 | 312.172 | 3.8165316 | 0.06167064 | |
| D1 | 3370.281 | 50.6055993 | 1.481113219 | |
| E1 | Nd | Nd | Nd | |
| F1 | Nd | Nd | Nd | |
| Beta-sitosterol | SAmple | B-sitosterol peak area | Beta-SITerol µg/ml | µg/gm dry powder of plant |
| A1 | 3327.305 | 194.64734 | 194.6473425 | |
| B1 | Nd | Nd | Nd | |
| C1 | Nd | Nd | Nd | |
| D1 | Nd | Nd | Nd | |
| E1 | Nd | Nd | Nd | |
| F1 | Nd | Nd | Nd | |
| *Nd=Not detected | ||||
Table 3: The conc. and peak area of Stigma sterol and Beta-sitosterol. At different parts, stages and type of propagated Iraqi Medicago sativa ordinary plant and tissue culture seeds in petroleum ether fractions.
RP-HPLC analysis for aqueous layer fractions
According to the RP-HPLC analysis of the six Iraqi Medicago sativa samples, Caffiec acid, chlorogenic acid, myricetin, gallic acid and Naringenine was the targeted analytes, in which caffeic acid and gallic acid present in all fractions while myricetin were present only in fraction B2, chlorogenic acid disappear from fraction E2 and Naringenine disappear from fraction F2, as apparent in Figure 4, showing the specific phenolic acids and flavonoids compound profiles from different Iraqi Medicago sativa samples.
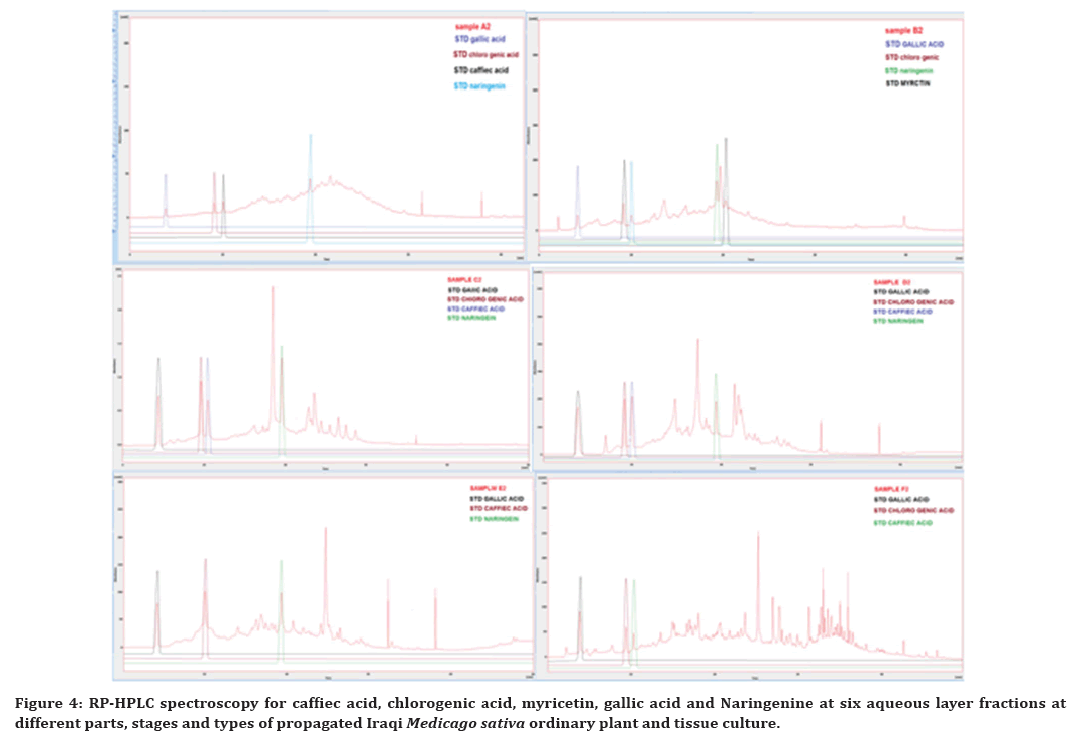
Figure 4. RP-HPLC spectroscopy for caffiec acid, chlorogenic acid, myricetin, gallic acid and Naringenine at six aqueous layer fractions at different parts, stages and types of propagated Iraqi Medicago sativa ordinary plant and tissue culture.
Concerning quantitative determination of Caffiec acid, chlorogenic acid, myricetin, gallic acid and Naringenine in the desired fractions serial dilutions of the standards were prepared. A plot of area vs concentration of Caffiec acid, chlorogenic acid, myricetin, gallic acid and Naringenine standards shows a linear fit which was subsequently used for the quantification of Caffiec acid, chlorogenic acid, myricetin, gallic acid and Naringenine in the analyzed fractions as illustrated in Table 4 and Figure 5.
| Phytochemicals | HPLC results | |||
|---|---|---|---|---|
| Caffeic acid | sample | CAFFIEC ACID peak area | caffiec acid µg/ml | µg/gm dry powder of plant |
| A2 | 170.319 | 2.7080721 | 0.043031266 | |
| B2 | 114.404 | 1.8190236 | 0.042482469 | |
| C2 | 347.101 | 5.5189059 | 0.107787911 | |
| D2 | 261.436 | 4.1568324 | 0.314199194 | |
| E2 | 77.543 | 1.2329337 | 0.157432804 | |
| F2 | 227.825 | 3.6224175 | 0.197057789 | |
| Chlorogenic acid | sample | chloro genic acid peak area | chloro genic acid µg/ml | µg/gm dry powdeplant |
| A2 | 158.832 | 2.4608688 | 0.039103205 | |
| B2 | 248.399 | 6.7959116 | 0.158715426 | |
| C2 | 1374.132 | 61.2813888 | 1.196866378 | |
| D2 | 217.238 | 5.2877192 | 0.399678636 | |
| E2 | Nd | Nd | Nd | |
| F2 | 139.504 | 1.5253936 | 0.082980686 | |
| Myricetine | sample | MYRCTIN Peak area | MYRCTIN µg/ml | µg/gm dry powder of plant |
| A2 | Nd | Nd | Nd | |
| B2 | 319.189 | 8.1712384 | 0.19083555 | |
| C2 | Nd | Nd | Nd | |
| D2 | Nd | Nd | Nd | |
| E2 | Nd | Nd | Nd | |
| F2 | Nd | Nd | Nd | |
| Gallic acid | sample | GALLIC ACID peak area | GALLIC ACID µg/ml | µg/gm dry powder of plant |
| A2 | 187.525 | 2.53395 | 0.040264466 | |
| B2 | 162.407 | 2.182298 | 0.050966578 | |
| C2 | 1116.323 | 15.537122 | 0.303450351 | |
| D2 | 267.665 | 3.65591 | 0.27633637 | |
| E2 | 193.497 | 2.617558 | 0.334234919 | |
| F2 | 75.662 | 0.967868 | 0.052651559 | |
| Naringinine | sample | Naringinin peak area | Naringinin µg/ml | µg/gm dry powder of plant |
| A2 | 270.98 | 4.400926 | 0.069930714 | |
| B2 | 269.01 | 4.373937 | 0.10215131 | |
| C2 | 1394.128 | 19.7880536 | 0.386473897 | |
| D2 | 120.99 | 2.346063 | 0.177330003 | |
| E2 | 159.865 | 2.8786505 | 0.367573715 | |
| F2 | Nd | Nd | Nd | |
Table 4: The conc. and peak area Of caffiec acid, chlorogenic acid, myricetin, gallic acid and naringinine. At 6 whole remaining fractions at different parts, stages and types of propagated Iraqi Medicago sativa ordinary plant and tissue culture seeds.
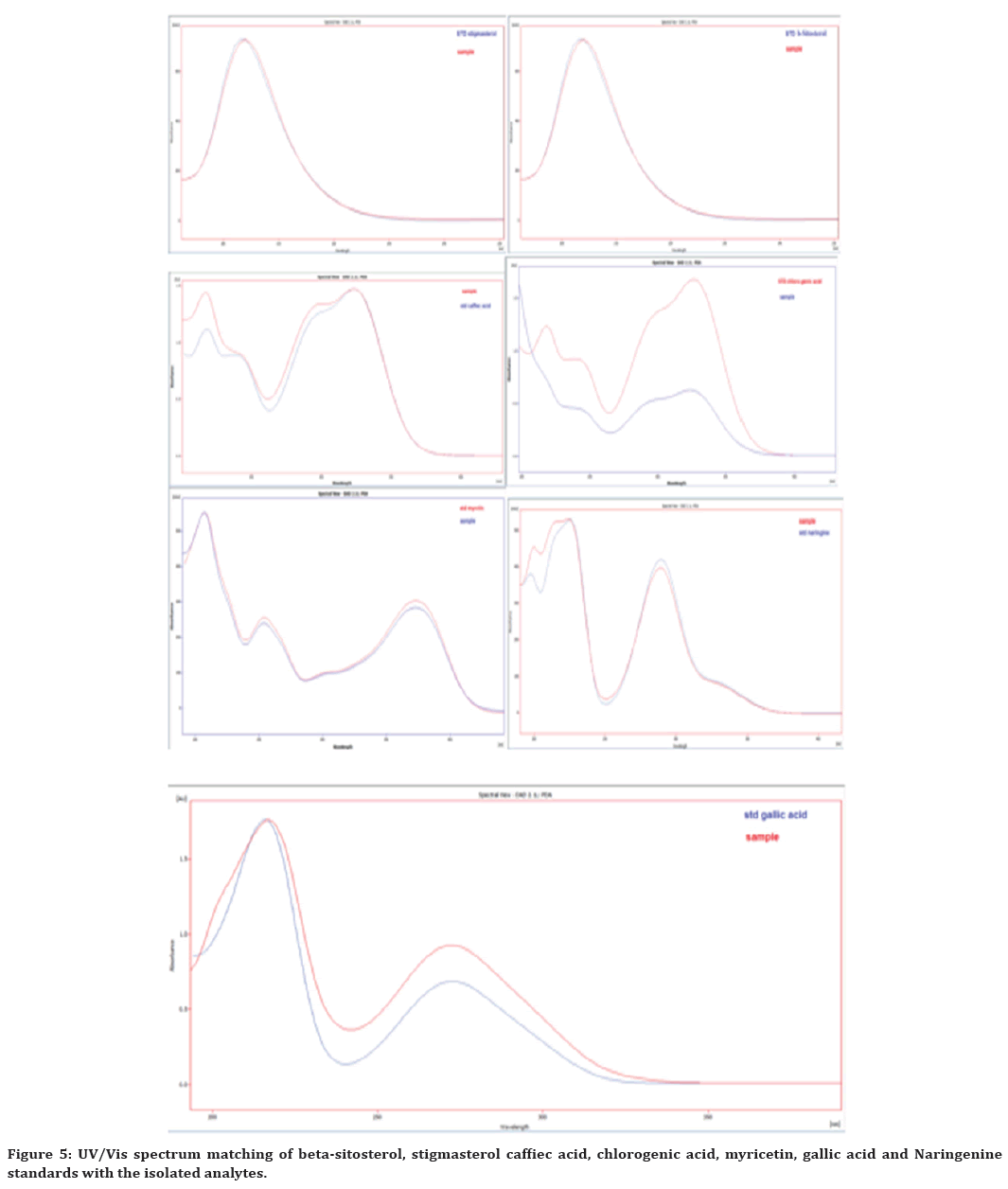
Figure 5. UV/Vis spectrum matching of beta-sitosterol, stigmasterol caffiec acid, chlorogenic acid, myricetin, gallic acid and Naringenine standards with the isolated analytes.
Comparative correlation between different fractions constituents
Comparison between different parts, stages and type of ordinary and tissue culture propagated Iraqi Medicago sativa relating on phytochemical concentrations yield by RP- HPLC (micro gram\gram of plant dry powder) for identified constituent steroids, phenolic acids and flavonoids.
Comparison between two different stages
Comparison between mature (fraction A) and un mature (fraction B) extracts of ordinary Iraqi Medicago sativa is illustrated in Table 5.1 revealing the following:
| Type | RP-HPLC concentrations results for each phytochemicals at different parts, stages of ordinary plant and type of tissue culture | |||||||
|---|---|---|---|---|---|---|---|---|
| I | Type | Betasitosterol | Stigmasterol | Caffiec acid | Chlorogenic acid | myricetin | Gallic acid | naringenine |
| A | 194.6473425 | 1.5819856 | 0.0430313 | 0.0391032 | Nd | 0.0402645 | 0.0699307 | |
| B | Nd | 0.3083286 | 0.0424825 | 0.1587154 | 0.1908356 | 0.0509666 | 0.1021513 | |
| II | Type | Betasitosterol | Stigmasterol | Caffiec acid | Chloro genic acid | myricetin | Gallic acid | naringenine |
| A | 194.6473425 | 1.5819856 | 0.0430313 | 0.0391032 | Nd | 0.0402645 | 0.0699307 | |
| C | Nd | 0.0616706 | 0.1077879 | 1.1968664 | Nd | 0.3034504 | 0.3864739 | |
| III | Type | Beta sitosterol | Stigma sterol | Caffiec acid | Chlorogenic acid | myricetin | Gallic acid | naringenine |
| A | 194.6473425 | 1.5819856 | 0.0430313 | 0.0391032 | Nd | 0.0402645 | 0.0699307 | |
| D | Nd | 1.4811132 | 0.3141992 | 0.3996786 | Nd | 0.2763364 | 0.17733 | |
| IV | Type | Beta sitosterol | Stigma sterol | Caffiec acid | Chlorogenic acid | myricetin | Gallic acid | naringenine |
| D | Nd | 1.4811132 | 0.3141992 | 0.3996786 | Nd | 0.2763364 | 0.17733 | |
| F | Nd | Nd | 0.1970578 | 0.0829807 | Nd | 0.0526516 | Nd | |
| V | Type | Beta sitosterol | Stigma sterol | Caffiec acid | Chlorogenic acid | myricetin | Gallic acid | naringenine |
| D | Nd | 1.4811132 | 0.3141992 | 0.3996786 | Nd | 0.2763364 | 0.17733 | |
| E | Nd | Nd | 0.1574328 | Nd | Nd | 0.3342349 | 0.3675737 | |
| *Nd=Not detected | ||||||||
Table 5: RP-HPLC concentrations results comparisons for each parts, stages and type of tissue culture employed of Iraqi Medicago sativa (µg/gm dry powder of plant).
✓ Steroids (Beta-sitosterol, stigmasterol) was affected by the maturation of the plant in which the mature plants have higher concentration than un mature.
✓ Caffiec acid concentration wasn’t affected by maturation, so the cut time have no effect on the concentration of caffiec acid.
✓ Chlorogenic acid was affected by the maturation, in which the un mature plant has higher concentration than the mature, so the maturation will affect the amount present in the plant.
✓ Myricetin present at the early stages of plant maturation and disappear at the maturation, so the maturation decreases its concentration [19].
✓ Gallic acid concentration wasn’t affected by the maturation of plant.
✓ Naringenine with higher concentration at the early stages of maturation of the plant, at which the concentration decrease in mature plant.
✓ The cut time have approved effect on the concentration of phytochemicals present in the ordinary propagated Iraqi Medicago sativa [20].
Comparison between two different parts
Comparison between top flowers (fraction C) extracts of mature plant and the aerial parts extracts (fraction A) of the same mature ordinary Iraqi Medicago sativa) is illustrated in Table 5.2 revealing the following.
✓ Steroids (beta-sitosterol, stigmasterol) will disappear or decreased at the tope flower of the mature plant.
✓ Caffiec acid, chlorogenic acid, gallic acid and Naringenine present with higher concentration at the top flower than the aerial parts of the mature plant.
✓ Myricetin disappear from the top flower and the aerial parts extracts.
✓ The top flower of the Iraqi Medicago sativa have appreciable conc. of phytochemical in comparison with the other aerial parts of the plant [21].
Comparison between two different propagations
Comparison between aerial part extracts of mature plant (fraction A) and the aerial parts extracts of the same mature seed tissue culture (fraction D) propagated at white light of Iraqi Medicago sativa is illustrated in Table 5.3 revealing the following:
✓ Steroids, beta- sitosterol wasn’t found in fraction D, stigma- sterol has the same conc. of A, so there is no benefit of doing tissue culture if the desire compounds were steroids.
✓ Caffiec acid, chlorogenic acid, gallic acid and Naringenine concentrations increased more than one double in fractions D than fraction A, so there is more benefit of doing tissue culture technique to get oriented increased concentration of them.
✓ Myricetin still absent at fraction A and D.
✓ The application of advanced techniques of propagation like tissue culture may give appreciable results in orientation to increase the phytochemicals concentrations desired like phenolic compounds and may open many gates to benefit from the secondary metabolites present in the plant [22].
Comparison between two different propagations types of tissue culture
Comparison between aerial part extracts of mature seed tissue culture (fraction D) propagated at white light and the same one but propagated at red light (fraction F) of Iraqi Medicago sativa is illustrated in Table 5.4 revealing the following.
There was no benefit of doing this technique (propagation at red light) to get more yield concentrations of phytochemical, in which there was a noticeable decrease or absence in concentration yield of phytochemicals.
Comparison between two different type of tissue culture
Comparison between aerial part extracts of mature seed tissue culture (fraction D) propagated at white light and the same one but propagated at different technique called callus tissue culture (fraction E) of Iraqi Medicago sativa is illustrated bellow in Table 5.5 revealing the following.
✓ There was no benefit of doing this technique (callus tissue culture) to get oriented increase concentrations of phytochemicals, in which some of them absent and the other still have the same concentration except Naringenine have double concentration in fraction E than fraction D.
✓ At this article, application of different techniques other than propagation at white light of tissue culture wasn’t gave a good results unless some changes in callus tissue culture may have an appreciation for other researchers at this plant or others [23].
Conclusion
The investigated Iraqi Medicago sativa which propagated by different techniques, such as soil propagation and tissue culture propagation, plant parts and time to harvest yielding different phytochemicals and suggest reasons for their varying chemical compositions. This is the first study in Iraq with results providing a comprehensive evaluation for the quality and quantity of some phytochemicals in ordinary Medicago sativa and tissue cultured ones, such as steroids, phenolic acids and flavonoids which play an important role in medicinal uses of these species over the past centuries, spreading all over the world and providing for Iraqi as do for worldwide researchers to have enormous and inexpensive sources of many phytochemicals required to produce nutraceuticals, antioxidants, and other therapeutic drugs.
The TLC and RP-HPLC help in qualification and quantification of different phytochemicals at different plant parts and types assisting in clearing the fog between tissue cultured and ordinary plants chemical constituents, although they have similar genetic information and homoplastic morphologies with increasing or decreasing some phytochemical existence and concentration.
Acknowledgements
✓ This research was supported by Collage of agriculture\University of Al Kufa\Department of Horticulture and Landscape Gardining\Horticulture Science (Promology-Tissue Culture) represented by Prof. Dr. Muslim Abd Ali Abdulhussein and Dhurgham Basim Naji.
✓ Special thanks to Al-Fadhil company for their supporting effort in doing the RP-HPLC results.
✓ Its agood chance to thank Dr. Hayder Obayes Hashim and Dr. Mudher Khudher Muhammed for their supporting and efforts in completing this article.
References
- Rafińska K, Pomastowski P, Wrona O, et al. Medicago sativa as a source of secondary metabolites for agriculture and pharmaceutical industry. Phytochem Lett 2017; 20:520–539.
- Gault RR, Peoples MB, Turner GL, et al. Nitrogen fixation by irrigated lucerne during the first three years after establishment. Aust J Agric Res 1995; 46:1401–1425.
- Lenné JM, Wood D. Is there a ‘logic of fodder legumes’ in Africa? Food Policy 2004; 29:565–585.
- Mielmann A. The utilisation of lucerne (Medicago sativa): A review. Br Food J 2013.
- Stinner DH, Glick I, Stinner BR. Forage legumes and cultural sustainability: Lessons from history. Agric Ecosyst Environ 1992; 40:233–48.
- Zubair HM, Pratley JE, Sandral GA, et al. Allelopathic interference of alfalfa (Medicago sativa L.) genotypes to annual ryegrass (Lolium rigidum). J Plant Res 2017; 130:647–658.
- Barnes DK. Alfalfa germplasm in the United States: Genetic vulnerability, use, improvement, and maintenance. Department of Agriculture, Agricultural Research Service 1977.
- Savangikar VA. Role of low cost options in tissue culture. In: Low cost options for tissue culture technology in developing countries Proceedings of a Technical Meeting organized by the Joint FAO/IAEA Division of Nuclear Techniques in Food and Agriculture. 2004; 11–15.
- Cannell JP. Natural products isolation Human Press. Totawa, New Jersy 1998; 560.
- Fan XH, Cheng YY, Ye ZL, et al. Multiple chromatographic fingerprinting and its application to the quality control of herbal medicines. Anal Chim Acta 2006; 555:217–224.
- https://bijps.uobaghdad.edu.iq/index.php/bijps/article/download/705/613
- Abdul-Jalil TZ. Screening of rutin from seeds and leaves extracts of dill, coriander and fennel cultivated in Iraq. Pharm Glob 2013; 4:1.
- Hamad MN. Detection and isolation of flavonoids from Calendula officinalis (F. Asteraceae) cultivated in Iraq. Iraqi J Pharm Sci 2016; 1–6.
- Raj D. Thin-layer chromatography with eutectic mobile phases—preliminary results. J Chromatogr A 2020; 1621:461044.
- Smith RM. Retention indices in reversed-phase HPLC. Adv Chromatogr 2021; 277–319.
- Lee DG, Lee J, Kim KT, et al. High-performance liquid chromatography analysis of phytosterols in Panax ginseng root grown under different conditions. J Ginseng Res 2018; 42:16–20.
- Seal T. Quantitative HPLC analysis of phenolic acids, flavonoids and ascorbic acid in four different solvent extracts of two wild edible leaves, Sonchus arvensis and Oenanthe linearis of North-Eastern region in India. J Appl Pharm Sci 2016; 6:157–166.
- Wakte PS, Sachin BS, Patil AA, et al. Optimization of microwave, ultra-sonic and supercritical carbon dioxide assisted extraction techniques for curcumin from Curcuma longa. Sep Purif Technol 2011; 79:50–55.
- Oh M, Rajashekar CB. Antioxidant content of edible sprouts: Effects of environmental shocks. J Sci Food Agric 2009; 89:2221–2227.
- Bora KS, Sharma A. Phytochemical and pharmacological potential of Medicago sativa: A review. Pharm Biol 2011; 49:211–220.
- Haq I. Safety of medicinal plants. Pak J Med Res 2004; 43:203–210.
- Adams M, Gmünder F, Hamburger M. Plants traditionally used in age related brain disorders—A survey of ethnobotanical literature. J Ethnopharmacol 2007; 113:363–381.
- Gray AM, Flatt PR. Pancreatic and extra-pancreatic effects of the traditional anti-diabetic plant, Medicago sativa (lucerne). Br J Nutr 1997; 78:325–334.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Author Info
Department of Pharmacognosy and Medicinal Plants, College of Pharmacy University of Baghdad, Baghdad, IraqReceived: 07-Mar-2022, Manuscript No. JRMDS-22-52364; Editor assigned: 09-Mar-2022, Pre QC No. JRMDS-22-52364 (PQ); Reviewed: 23-Mar-2022, QC No. JRMDS-22-52364; Revised: 28-Mar-2022, Manuscript No. JRMDS-22-52364 (R); Published: 04-Apr-2022