
Advances in Pediatric Research
Open Access
ISSN: 2385-4529

ISSN: 2385-4529
Case Report - (2023)Volume 10, Issue 2
Hemophagocytic Lymphohistiocytosis (HLH) is a rare, hyperinflammatory syndrome due to pathologic immune activation. HLH in the neonatal period is extremely rare. We present the case of a critically ill newborn full-term via urgent cesarean delivery for concerns for hydrops fetalis, ultimately found to have familial HLH. HLH carries a high fatality rate and poor prognosis. A high clinical suspicion of HLH and early initiation of treatment is crucial to survival. In cases where no amount of medical treatment is likely to be more than palliative, early discussions of goals of care is imperative to avoid unnecessary treatments.
Hemophagocytic lymphohistiocytosis; Hydrops fetalis; Non-immune hydrops fetalis; Neonatal enteroviral infection
Hemophagocytic lymphohistiocytosis (HLH) is a rare, lifethreatening state of extreme inflammation and tissue destruction. HLH can occur in the presence of genetic mutations or secondary to infectious, inflammatory, or neoplastic triggers [1,2]. HLH presents similarly to sepsis [3]. Key clinical findings include high fevers, cytopenias, and hepatosplenomegaly [2]. HLH is typically aggressive and can be fatal. A high clinical suspicion of HLH and early initiation of treatment is crucial to survival. We present a case of familial HLH in a newborn to add to the existing literature and increase awareness of this rare condition.
A baby girl was born at 37 weeks by urgent cesarean delivery due to concerns for hydrops fetalis on prenatal ultrasound. Maternal history is significant for hypothyroidism on levothyroxine. Prenatal labs were significant for positive Group B Streptococcus and maternal blood type is B+. The pregnancy was otherwise uncomplicated. There was no consanguinity reported in the parents and this is their first child. Rupture of membranes at delivery was clear and Appearance, Pulse, Grimace, Activity, and Respiration (APGAR) scores were 7 and 8 at 1 and 5 minutes, respectively. Immediately after birth, respiratory distress was noted, requiring continuous positive airway pressure and positive pressure ventilation. Subsequently, the newborn required intubation with mechanical ventilation and pressure support. In the NICU, blood cultures were obtained, and the newborn was started on empiric antibiotics. Initial echocardiogram showed a patent ductus arteriosus and atrial septal defect. The newborn was soon found to have hepatic and renal dysfunction due to coagulopathy and oliguria, respectively; significant metabolic acidosis and hypoglycemia requiring sodium bicarbonate and dextrose administration, respectively; and anemia and thrombocytopenia requiring transfusions.
The newborn is referred to our center for further evaluation and management. Upon arrival, she is ill-appearing with anasarca, liver failure with transaminitis and direct hyperbilirubinemia, as well as signs of disseminated intravascular coagulation such as thrombocytopenia, elevated partial thromboplastin time and prothrombin time, increased levels of plasma D-dimers, decreasing plasma fibrinogen level, and symptomatic bleeding. Due to ongoing acute kidney injury and oliguria, a foley catheter was placed and the newborn was started on diuretics. An extensive metabolic work-up was also initiated, including but not limited to, blood lactate with simultaneous pyruvate, blood amino acids, blood acylcarnitines, urine organic acids, whole exome sequencing, and mitochondrial DNA testing. The newborn remained on respiratory support and total parenteral nutrition due to inability to tolerate feeds. Antibiotics were discontinued after multiple negative blood cultures. Of note, Cerebrospinal Fluid (CSF) cultures were unable to be obtained due to newborn’s clinical instability. Parvovirus, Toxoplasmosis, and Cytomegalovirus (CMV) studies were also negative. After consultation with the infectious disease service, neonatal enteroviral infection became a leading consideration. The team attempted to obtain emergency Food and Drug Administration (FDA) approval for investigational drug, Pocapivir, if positive, but enterovirus Polymerase Chain Reaction (PCR) was ultimately negative.
After consultation with the hematology service, a work-up for hemophagocytic lymphohistiocytosis (HLH) was initiated and the newborn was started on Anakinra prophylactically. By the third day of life, the newborn met five of the eight Histiocyte Society HLH-2004 diagnostic criteria: bicytopenia, splenomegaly, hyperferritinemia, hypofibrinogenemia, and elevated Soluble Interleukin-2 Receptor (sIL-2R) [4]. Subsequently, the anakinra dose was increased, and the newborn was started on dexamethasone. The following day, the newborn was found to have compound heterozygous mutations in the PRF1 gene, consistent with familial HLH. After the diagnosis of HLH was confirmed on the genetic panel, the newborn was started on targeted therapy with gamifant and then etoposide was added as well.
Unfortunately, all treatment options were exhausted, and the newborn was not a transplant candidate due to her clinical status. The newborn had ongoing difficulty with ventilation and oxygenation and was intermittently on pressure support. Finally, the newborn developed pulmonary hemorrhage in the setting of severe thrombocytopenia and coagulopathy and was placed on an oscillator. Head ultrasounds showed bilateral intraparenchymal hemorrhages with effacement of the right ventricle and midline shift. Unfortunately, the newborn began to deteriorate in the following days. The newborn ultimately succumbed to cardiopulmonary arrest secondary to severe metabolic acidosis and end organ system failure (Figure 1).
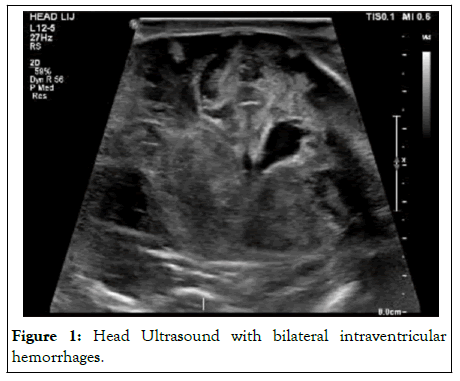
Figure 1: Head Ultrasound with bilateral intraventricular hemorrhages.
Hemophagocytic lymphohistiocytosis (HLH) is an aggressive and life-threatening syndrome of excessive inflammation and tissue destruction due to abnormal immune activation. An uncontrolled immune response occurs due to disordered cytotoxic CD8+ T-cell activation in the absence of normal downregulation by activated macrophages and lymphocytes [1]. This leads to the overproduction of inflammatory cytokines including Interleukin-2 (IL-2), IL-6, IL-10, IL-8, and interferongamma [1]. The resulting cytokine storm and subsequent accumulation of lymphohistiocytic infiltrates has detrimental effects on multiple organ systems [5].
HLH can be primary or secondary to triggers such as infection, malignancy, autoimmune disease, immunodeficiency, and drugs. It is most often triggered by an infectious agent with ebstein barr virus and Cytomegalovirus (CMV) being most common [6]. In the neonatal period, Herpes Simplex virus is the most common cause of secondary HLH [7]. In comparison, familial or primary HLH is far less common. Familial HLH is associated with genetic mutations that can be autosomal recessive or X-linked [1,5]. The Perforin gene (PRF1) is the most common gene mutation associated with familial HLH. Several gene mutations have been identified as genetic causes of predisposition to HLH, including UNC13D, STXBP1, RAB27A, STX11, SH2D1A, and XIAP [2]. Deficiencies in these genes lead to abnormal cytotoxic function [5]. Onset is typically during infancy or early childhood and is associated with a significantly higher mortality rate [2]. If left untreated, the median survival is less than a few months [7].
Key clinical findings of HLH include high fevers, cytopenias, and hepatosplenomegaly. Other findings include coagulopathy, transaminitis, hypertriglyceridemia, hypofibrinogenemia, and elevated ferritin. Histopathology reveals hemophagocytosis in the spleen, lymph nodes, bone marrow, and liver [4]. The diagnostic criteria proposed by the Histiocyte Society HLH-2004 study reflect the extreme inflammation of HLH [4]. However, these findings are generally late findings of disease progression. Due to the nonspecific findings that often rapidly progress to multiorgan dysfunction, it is common for HLH to be misdiagnosed as sepsis [3]. Thus, HLH should be included in the differential diagnosis of sepsis and multiorgan dysfunction. Reports of HLH diagnosed in the neonatal period presented with hydrops fetalis, fulminant liver failure, respiratory distress, thrombocytopenia, sepsis, and multiorgan failure [5,7-10]. Research also corroborates the overlapping clinical and laboratory findings in neonatal enteroviral infection and HLH in the neonatal period, making the diagnosis difficult to distinguish.
Histiocyte society HLH-2004 diagnostic criteria
The diagnosis of HLH requires at least 1 of the following:
A molecular diagnosis consistent with HLH: Pathological mutations of PRF1, UNC13D, STXBP1, RAB27A, STX11, SH2D1A, or XIAP.
At least 5 of the following diagnostic criteria are fulfilled: Fever ≥ 38.5°C, splenomegaly, cytopenias affecting at least 2 cell lineages in the peripheral blood includes hemoglobin <90 g/L or hemoglobin <100 g/L in newborn <4 weeks, platelets <100 × 109/ L, neutrophils < 1.0 × 109/L, hypertriglyceridemia and/or hypofibrinogenemia includes fasting triglycerides ≥ 3.0 mmol/L, Fibrinogen ≤ 1.5 g/L; Hemophagocytosis in bone marrow or spleen or lymph nodes or liver, Low or absent natural killer cell activity, Ferritin 500 mg/ L, Soluble CD25 (i.e., soluble IL-2 receptor) 2 400 μ/mL.
It is important to emphasize the critical need to start treatment once HLH is suspected. This is because the early disease progression of HLH may not yet fulfill the diagnostic criteria. The diagnostic criteria reflect the inherited susceptibility to HLH, immune activation, and immunopathology. The criteria were established to help guide the diagnosis of HLH. However, clinicians should be empowered to use clinical judgement and treatment should not be withheld based on this criterion if HLH is suspected, given the time it takes to receive some of these results. Finally, given the poor prognosis associated with HLH, family discussions should be initiated early.
Treatment for HLH involves suppressing the life-threatening inflammation. The goal of treatment of HLH is to dampen the cytokine storm, thereby decreasing inflammation. Treatment often consists of immunosuppressive therapy as well as chemotherapy with dexamethasone, Etoposide, and cyclosporine A4. Research shows chemo-immunotherapy can prolong survival. etoposide, however, is reserved for severe or refractory disease due to side effects [6]. Anakinra, an interleukin-1 receptor antagonist, is a novel drug that has further improved the prognosis of HLH, when initiated early [11,12]. Emapalumab, a human monoclonal antibody against interferongamma, was recently approved for the treatment of familial HLH and has been shown to increase survival [13]. Nonetheless, hematopoietic stem cell transplantation offers the best chance at survival and is often necessary in familial HLH and refractory HLH [4].
This case highlights the importance of clinical suspicion of rare diagnoses, such as HLH. HLH is extremely rare in the neonatal period and may present with non-immune hydrops fetalis and neonatal liver failure, as seen in this case. Neonatal HLH can also be difficult to distinguish from neonatal enteroviral sepsis. HLH, and especially familial HLH when diagnosed in the newborn period, carries a grave prognosis. A high clinical suspicion of HLH and early initiation of treatment is crucial to survival. Unfortunately, this case was advanced despite early diagnosis and treatment. Family discussions regarding goals of care should be initiated early, and where possible a medical ethics and palliative care consult may be helpful.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Kullmann KC (2023) Effects of Hemophagocytic Lymphohistiocytosis in Newborns. Adv Pediatr Res. 10:055.
Received: 04-May-2023, Manuscript No. LDAPR-23-23890; Editor assigned: 08-May-2023, Pre QC No. LDAPR-23-23890 (PQ); Reviewed: 22-May-2023, QC No. LDAPR-23-23890; Revised: 29-May-2023, Manuscript No. LDAPR-23-23890 (R); Published: 05-Jun-2023 , DOI: 10.4172/2385-4529.23.10.055
Copyright: © 2023 Kullmann KC. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.