
Advances in Pediatric Research
Open Access
ISSN: 2385-4529

ISSN: 2385-4529
Research - (2021)Volume 8, Issue 2
Background: While several parameters of fluid responsiveness have been validated in adults, they cannot be directly applied in children primarily because of the pediatric specificities of the cardiovascular system. Respiratory variation of Aortic Blood Flow Peak Velocity (ΔVpeak) has been promoted as an accurate predictor of fluid responsiveness in children. Therefore, we aim at characterizing the potential impact of optimization of intraoperative hemodynamic condition using goal directed fluid and hemodynamic therapy (GDFHT based on ΔVpeak assessed by transthoracic echocardiography on postoperative outcome in children.
Methods: Children aged less than 18 years old and admitted for major non cardiac surgery will be eligible. After obtaining parental consent, children will be randomized into two groups: Group GD, where fluid and hemodynamic therapy will be guided with ΔVpeak and Group SC, with fluid and hemodynamic therapy managed according to routine. Primary outcome will be postoperative morbidity until 30 days postoperatively defined as surgical and/ or organ failure. Secondary outcome will include length of stay in the intensive care unit, length of mechanical ventilation and length of hospital stay. Based on the primary end-point, 400 patients are required in order to have a significant difference between the two groups.
Results and Conclusion: This multicentre randomized controlled trial will clarify the impact of GDFHT based on ΔVpeak assessed by transthoracic echocardiography on postoperative outcomes in children undergoing elective or urgent major non-cardiac surgery.
A recent retrospective observational study was conducted to determine predictive factors of adverse postoperative outcome in pediatric patients in neurosurgery, abdominal and orthopedic surgery [1]. This observational retrospective pediatric study of 594 children revealed that ASA status (ASA 3 or more) was the independent predictive factor for mortality. ASA status (ASA 3 or more) was an independent predictive factor of postoperative morbidity (in terms of organ dysfunction) among other factors (transfusion, emergency situations, type of surgery and age). The incidence of postoperative morbidity in terms of complications was 23% in this study with ASA status score 3 or more being one of the predictive factors among five others. In adults, a systematic review and meta-analysis demonstrated that intraoperative goal directed fluid and hemodynamic therapy (GDFHT) in high risk patient’s improved postoperative outcome in terms of mortality and morbidity [2]. In children, the impact of GDFHT on postoperative outcome has not yet been evidenced. A recent systematic review and meta-analysis in more than 3000 pediatric cardiac surgical patients identified unoptimal intraoperative lactate levels, cerebral, renal regional oxyen saturations and central venous oxygen saturation as predictive parameters of postoperative adverse outcome in terms of mortality and morbidity [3]. These altered parameters may reflect unoptimal tissular perfusion which depends on oxygen delivery which depends among others on cardiac output [4]. The aim of GDFHT is to improve tissular perfusion by optimizing cardiac output. Unoptimal tissular perfusion with insufficient or plethoric fluid therapy can lead to organ dysfunction. The issue is how to improve postoperative outcome in pediatric patients with an ASA 3 or more status in major surgery. GDFHT applied in adults cannot be directly extrapolated to the pediatric population because validated parameters in children for GDFHT are necessary. The hypothesis is by optimizing intraoperative management with GDFHT in high risk pediatric patients in major surgery, postoperative outcome in terms of morbidity will be improved. Since postoperative morbidity in terms of complications is multifactorial as precised here above, with ASA status being predictive in 1/5 of the multiple risk factors, the postoperative complications rate would be reduced by 5% or more (will decrease from 23% to 18% or less at least).
Evidence concerning validated parameters of fluid responsiveness in children is increasing.
Fluid responsiveness is an important issue in surgical and critical ill pediatric patients. Several indices have been studied in children with the aim to determine those which are suitable in pediatric patients. Parameters like pulse pressure variation (PPV) to assess fluid responsiveness remain until now controversial in children [5-8]. One important aspect is to have a non invasive tool or monitor to determine these parameters. A possible non invasive tool is trans-thoracic echocardiography. An indice like transthoracic aortic blood flow peak velocity variation (ΔV peak) has been validated as an accurate parameter of fluid responsiveness in ventilated children in several trials [5-8]. Since this indice has been validated to predict fluid responsiveness in several pediatric studies, it will be integrated in this prospective randomized controlled trial (RCT) to determine the impact of intraoperative goal directed fluid and hemodynamic therapy (GDFHT) with this parameter on postoperative outcome in high risk pediatric patients in major surgery. Another non invasive indice which has been validated in children is trans-thoracic aortic blood flow velocity time integral (VTI) which reflects stroke volume and cardiac output. Since cardiac output in children is heart rate dependent, distance minute (DM) which is obtained by multiplying VTI with heart rate (HR) is more accurate to assess cardiac output [9]. Stroke volume can be calculated with this formular VTI×D2×π/4 [7]. Cardiac output (CO) can be calculated with the following formular CO=VTI × Aortic annulus area × HR=VTI×D2×π/4×HR [10]. D is the aortic annulus diameter and HR the heart rate. ΔVpeak is calculated as follows [8]: [(Vpeak maximum-Vpeak minimum)/(Vpeak maximum+Vpeak minimum)/2]×100.
ΔVpeak and VTI will be assesed in the apical five chamber view and D will be assessed in the parasternal long axis view.
The primary objective of this study is to determine the impact of GDFHT with ΔVpeak with echocardiography on postoperative outcome in terms of morbidity. The secondary outcome is to determine the impact of GDFHT with ΔVpeak with echocardiography on postoperative length of stay in the intensive care unit (LOSICU), post-operative length of invasive or non invasive mechanical ventilation (LMV), postoperative length of hospital stay (LOS), intraoperative fluid therapy and intraoperative vasopressor inotropic therapy.
The primary outcome measures will be postoperative organ dysfunction until discharge from hospital. Organ dysfunction which will include infections will be defined according to the organ by combining clinical, biological and imaging findings.
The secondary outcome measures will be the number of postoperative days spent in the intensive care unit (ICU), the number of post-operative days spent on invasive or non invasive mechanical ventilation, the number of post-operative days spent in the conventional hospitalization ward, the quantity of intraoperative fluid therapy and vasopressor-inotropic score.
Four similar studies have been elaborated to determine hemodynamic echocardiographic parameters predictive of postoperative outcome and to determine their impact in goal directed fluid and hemodynmic therapy on postoperative outcome in children [11-14].
This study and the other four studies [11-14] are part of the Thesis entitled ‘‘Do goal directed therapies improve postoperative outcome in children? [15-18] (Perioperative goal directed fluid and hemodynamic therapy; transfusion goal directed therapy using viscoelastic methods and enhanced recovery after surgery and postoperative outcome) [19].
This study has been registered at the French National Agency on Medication and Drug Safety (ANSM, Agence Nationale de Sécurité du Médicament et des Produits de Santé) under the number ID RCB: 2020-A00214-35. After approval by the Ethics Committee and declaration at the CNIL, Commission Nationale de l’informatique et des Libertés, National Commission for Computer Science and Liberties, and parents informed and written consent, patients will be randomized in two groups in stratification (to obtain the same number of patients from different surgical specialities in the two groups, experimental and control) and single blinded. The experimental group is defined as the group where fluid and hemodynamic therapy is guided with transthoracic echocardiography precisely with ∆Vpeak. The control group is defined as the group where fluid therapy is guided according to the discretion of the medical doctor in charge or according to the local protocol if any exists.
Inclusion criteria are patients under 18 years old with ASA status II or more admitted for major surgery or major interventional radiology:
Neurosurgery: Craniosynostosis, cerebral tumors
Abdominal surgery laparoscopy or laparotomy: Abdominal tumors and masses, renal transplantation, hepatic transplantation, intestinal exeresis etc.
Thoracic surgery thoracoscopy or thoracotomy
Gynecologic surgery laparoscopy or laparotomy
Urologic surgery laparoscopy or laparotomy
Vascular surgery
Orthopedic surgery: Limb tumors, femoral and pelvic osteotomy, pelvic fracture, femoral diaphysal fracture, tibial diaphysal fracture, scoliosis if anterior approach etc.
Maxillo-facial, otorhinolaryngologic and plastic surgery: Lefort, exeresis of geant naevus, skin graft (transplantation) etc…
Polytrauma
Interventional radiology (angioembolisation etc….)
Exclusion criteria are patients more than 18 years old, cardiac surgery (cardiac patients will be investigated in a study apart), ASA status 1 and 2 (non-major surgery), parents or patient’s refusal, ventral decubitus (for practical reasons).
Fluid therapy in patients, in the experimental group will be directed with ∆Vpeak and DM (Figure 1) goal directed fluid and vasoactive inotropic therapy administration.
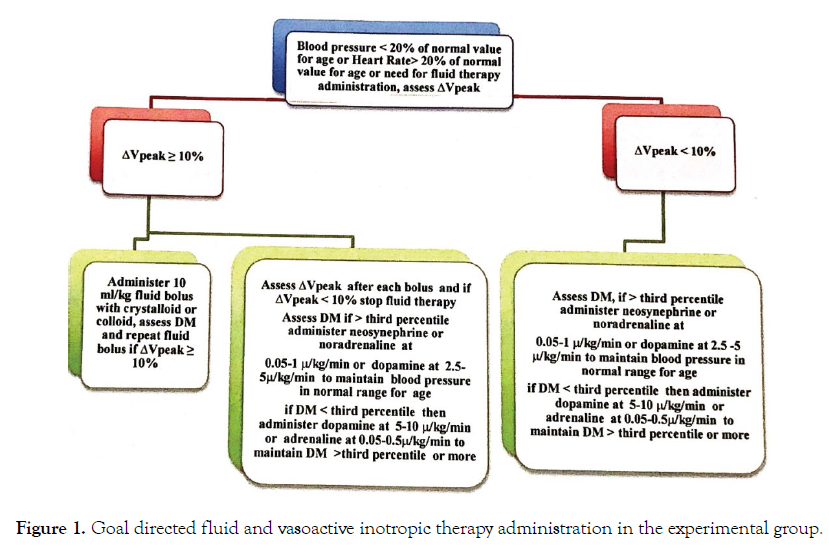
Figure 1: Goal directed fluid and vasoactive inotropic therapy administration in the experimental group.
Whenever a patient needs fluid administration on a basis of hypotension (decrease in more than 20% from the normal values for age) or tachycardia (more than 20% increase in heart rate from the normal values for age) or whenever the medical doctor in charge needs to give fluids, ∆Vpeak and DM will be assessed intraoperatively before fluid administration. If ∆Vpeak is more than 10%, a fluid bolus of 10 ml/kg with Ringer’s Lactate or Sodium Chloride 0.9% (NaCl 0.9%) or albumine 4% or hydroxylethylstarch (except in patients with sepsis or septic choc or renal impairement or coagulation disorders and in neonates) will be administered. After each 10 ml/kg bolus of crystalloid or colloid, ∆Vpeak and DM will be assessed.
∆Vpeak and DM will be measured in the apical five chamber view and will be calculated on three respiratory cycles. The effectiveness of fluid therapy will be considered if an increase in more than 10% of DM is observed. Fluid therapy will be suspended or not administered if ∆V peak is less than 10% and a vasoactive or inotropic drug will be administered as follows. If ∆Vpeak is less than 10% and DM is higher than the third percentile for age, administer neosynephrine or noradrenaline at 0.05-1 μ/kg/min or dopamine at 2.5-5 to maintain the blood pressure in normal range values for the age. If ΔVpeak is less than 10% and DM less than the third percentile for age, administer dopamine at 5-10 μ/ kg/min or adrenaline at 0.05-0.5μ/kg/min to maintain blood pressure in normal range values for age and maintain DM higher than the third percentile for age. Assess regularily (hourly if the patient’s state is stable) ΔVpeak and DM and repeat fluid boluses if necessary as recommended here above.
Since echocardiography is operator dependent, it will be realized by the same person experienced in echocardiography to reduce the inter-observer variability. The control group will be managed intraoperatively as usual at the discretion of the medical doctor in charge.
The induction and maintenance of anesthesia will be the same in the two groups: inhalational or intravenous anesthesia for induction and or maintenance. Pain managment will be according to the local protocol and should be similar in the two groups.
Transfusion management should be the same in the two groups according to local protocol if any exists.
Parameters such as age, gender, type of surgery, elective or urgent surgery, American Society of Anesthesiologists status (ASA), weight, height, prematurity, blood pressure, heart rate, pulse oxymetry, hemoglobin levels, platelet count, leucocyte count, activated thromboplastin, prothrombin time, fibrinogen, blood urea nitrogen, serum creatinin levels, C-reative protein levels (CRP), procalcitonin (PCT) levels, hepatic functional tests will be registered (if analysed).
Pre-operatively basal values of blood pressure, heart rate, core temperature, pulse oxymetry, will be registered prior to anesthesia and surgery and intraoperatively hourly. Intraoperative parameters registered will be blood product transfusion (packed red blood cells (PRBC), fresh frozen plasma (FFP), concentrated platelet units (CUP), fibrinogen, cryoprecipitate, concentrated complex of prothrombin (CPP) or other blood product derivatives, crystalloids and colloids or other fluids administered, blood loss, urinary output, quantity of inotrops administered and mechanical ventilation parameters, regional cerebral oxygen saturation, renal oxygen saturation and mixed venous oxygen saturation (if monitored) and lactate levels (if monitored). Postoperative parameters registered will be blood pressure, heart rate, core temperature, pulse oxymetry, mixed venous oxygen saturation (ScVO2), lactate levels, cerebral (ScO2) and renal oxygen saturation (SrO2), blood product transfusion (PRBC, FFP, CUP), fibrinogen, cryoprecipitate, concentrated complex of prothrombin other blood product derivatives, crystalloids, colloids or other fluids administered, blood loss, urinary output, quantity of inotrops admnistered, mechanical ventilation parameters, hemoglobin, platelet, leucocyte levels, CRP, PCT, hepatic functional tests, blood urea nitrogen, serum creatinin levels (if monitored and analysed); the presence or the absence of organ dysfunction, the number of days spent in the intensive care unit, under invasive and non invasive mechanical ventilation and the number of days spent in the standard hospitalization ward.
Statistic analysis will be realized with XLSTAT 2019.4.2 or plus software. Normally distributed and none normally distributed variables will be compared using Student t test or Mann-Whitney test and Wilcoxon test or Kruksal-Wallis test respectively. Normally distributed variables will be expressed in terms of means with standard deviation. None normally distributed variables will be expressed in terms of medians with interquartile ranges. Categorical variables will be compared with the exact Fisher’s test or Chi squarred test accordingly. Categorical variables will be expressed as percentages with 95% confidence intervals. To assess for independent predictors of adverse postoperative outcome, multivariate analysis will be realized. A p-value ≤ 0.05 will be considered significative. Missing data will not be included. The number of patients included will be 400 with 200 in each group. This number was calculated to assess for a significative difference between the two groups using the case control Chi squared test with Yates continuity correction. The study will be multicentric.
This study protocol was designed to describe the trial which will clarify the impact of Aortic Blood Flow Peak Velocity Variation with echocardiography in GDFHT on post-operative outcome in terms of morbidity, LOSICU, LMV, LOS, intraoperative and post-operative fluid therapy and vasopressor-inotropic therapy in pediatric patients admitted for major surgery or major interventional radiology.
Citation: Kumba C (2021) Goal directed fluid and hemodynamic therapy and postoperative outcomes in children: Value of trans-thoracic echocardiographic aortic blood flow peak velocity variation: A multicentre randomized controlled trial protocol. Adv Pediatr Res 7:35.
Received: 26-Jan-2021 Accepted: 28-Feb-2021 Published: 06-Mar-2021 , DOI: 10.35248/2385-4529.21.8.57
Copyright: © 2021 Kumba C. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.