Annals and Essences of Dentistry
Open Access
ISSN: 0975-8798, 0976-156X
ISSN: 0975-8798, 0976-156X
Short Communication - (2022)Volume 14, Issue 6
Periodontal disease is an inflammatory disorder caused by bacterial infection. Porphyromonas gingivalis (P. gingivalis) is often isolated from localized sites of periodontal tissue in patients with chronic periodontitis. Along with Tannerella forsythia and Treponema denticola, it is thought to be a causative bacterium of chronic periodontitis [1]. P. gingivalis is known to produce biofilms and gingipains, Arg-gingipain (Rgp) and secreting-type Rgp and Lys-gingipain (Kgp) [2], which are thought to be intimately linked with the pathology of periodontal disease.
Microbes use quorum sensing as a survival strategy. When microbes recognize autoinducers (information transmission substances), they induce gene expression in a density-dependent manner, resulting in the expression of phenotypes beneficial to their survival. Reportedly, biofilm formation and gingipains production by P. gingivalis are related to quorum sensing mediated by AutoinduceR-2 (AI-2) [1,3-8]. Hence, it is possible to suppress biofilm formation and gingipains production by inhibiting AI-2 in P. gingivalis. Hinokitiol is an active ingredient of quasi-drugs in Japan for periodontal disease. Owing to its antiseptic properties, hinokitiol has been demonstrated to prevent periodontal disease. In this study, we examined the hypothesis that hinokitiol inhibits AI-2, which is involved in quorum sensing in P. gingivalis. And we also verified that hinokitiol would suppress biofilm formation and gingipains production of P. gingivalis.
AI-2 inhibition by hinokitiol
To clarify how hinokitiol affects quorum sensing in P. gingivalis, we performed an AI-2 bioassay using V. harveyi ATCC BBA-1121. After mixing V. harveyi solution with hinokitiol solution or the control solution of AI-2 inhibitor, 4-bromo-5-(4-methoxyphenyl)-2(5H)-furanone (BMF) [9], the mixed solution was incubated for 10 minutes. Subsequently, the control solution of AI-2, 4-hydroxy-5-methyl-3-furanone (HMF) [10] or P. gingivalis ATCC 33277 culture filtrate was added and incubated for 4 hours. The luminescence from V. harveyi was measured using a luminometer. Because hinokitiol exhibits bacteriostatic and bactericidal properties at concentrations as low as 10 mM [11], lower concentrations of hinokitiol were used in the experiment to avoid bacteriostatic and bactericidal effects against V. harveyi. The results revealed that 200-300 μM hinokitiol inhibited AI-2 (P<0.05) (Figure 1) [12]. Moreover, 100 μM hinokitiol inhibited luminescence from V. harveyi, which was possibly induced by P. gingivalis AI-2 (P<0.05) (Figure 2) [12]. The results suggest that hinokitiol inhibits quorum sensing via P. gingivalis AI-2.
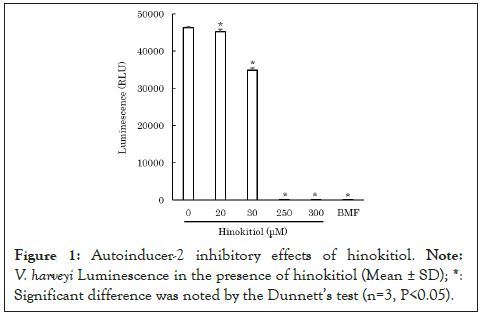
Figure 1: Autoinducer-2 inhibitory effects of hinokitiol. Note: V. harveyi Luminescence in the presence of hinokitiol (Mean ± SD). *: Significant difference was noted by the dunnett's test (n=3, P<0.05).
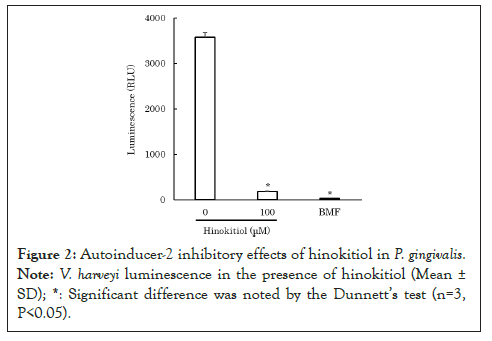
Figure 2: Autoinducer-2 inhibitory effects of hinokitiol in P. gingivalis. Note: Vibrio harveyi luminescence in the presence of hinokitiol (Mean ± SD). *: Significant difference was noted by the Dunnett's test (n=3, P<0.05).
Suppression of biofilm formation of P. gingivalis by hinokitiol
According to previous reports, AI-2 promotes biofilm formation in P. gingivalis [1,3-5]. Therefore, we examined the hypothesis that hinokitiol suppresses biofilm formation by inhibiting AI-2 in P. gingivalis. The biofilm assay for P. gingivalis was performed in accordance with the study by Qi et al. [13]. Briefly, biofilms of P. gingivalis was cultured with hinokitiol in a 96-well plate for 24 hours. After the culture, the wells were washed with distilled water, and the biofilms formed on the bottom surfaces of the wells were stained with crystal violet solution. After staining biofilms, the wells were washed with distilled water to remove unbound crystal violet from the biofilms. The staining solution was washed off the biofilms by pipetting 33% acetic acid aqueous solution into each well. The absorbance of the staining solution at 570 nm was measured to evaluate the amount of biofilms formed. The amount of biofilms formed was measured at low concentrations of 70-100 μM to avoid the bacteriostatic and bactericidal effects of hinokitiol on P. gingivalis. The results revealed that in the presence of hinokitiol, the amount of biofilms formed by P. gingivalis decreased significantly in a concentration-dependent manner (P<0.05) (Figure 3) [12]. During the measurement of biofilm formation quantity, P. gingivalis viable bacteria were counted, and the number of viable bacteria was equivalent to the control. This suggests that the reduction in biofilm formation in P. gingivalis is not due to the bactericidal action of hinokitiol.
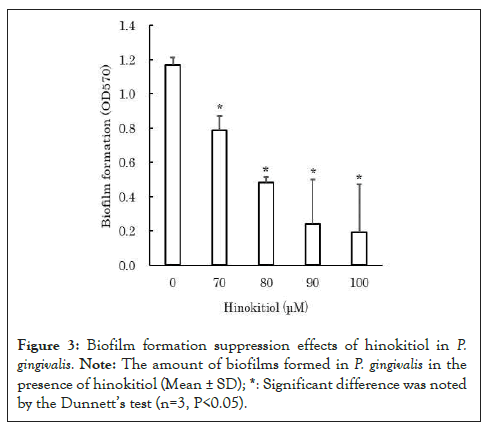
Figure 3: Biofilm formation suppression effects of hinokitiol in P. gingivalis. Note: The amount of biofilms formed in P. gingivalis in the presence of hinokitiol (Mean ± SD); *: Significant difference was noted by the Dunnett's test (n=3, P<0.05).
Improvement in the bactericidal efficacy of hinokitiol against P. ginigivalis after the suppression of biofilm formation
It is known that microbes increase their resistance to antimicrobial agents by forming biofilms [14,15]. In general, biofilm-forming microbes increase the expression of drug-resistant genes while decreasing cell metabolism [4]. Additionally, biofilms also act as barriers, preventing the permeation of bactericidal agents. Therefore, bactericidal agents are less effective against P. gingivalis within biofilms. When P. gingivalis forms biofilms within the oral cavity, it becomes more resistant to bactericidal agents. This is considered to be one of the factors involved in the development and progression of periodontitis. If the suppression of biofilm formation by P. gingivalis increases susceptibility to bactericidal agents, this may be used to prevent the development and progression of periodontitis. As hinokitiol was found to suppress the biofilm formation (Figure 4) [12], we comparatively evaluated the bactericidal efficacy of hinokitiol against nonbiofilm and biofilm-forming P. gingivalis bacteria. Hinokitiol solution was added to the biofilms prepared and to the floating bacteria solution until the final concentration of 4,000-6,000 μM was reached. After left for 30 minutes, the number of viable bacteria was counted for each. The results revealed that hinokitiol was more effective against nonbiofilm-forming P. gingivalis than biofilm-forming P. gingivalis bacteria. These results suggest that hinokitiol enhances its bactericidal efficacy by preventing P. gingivalis from forming biofilms, which further allows it to act more effectively against P. gingivalis.
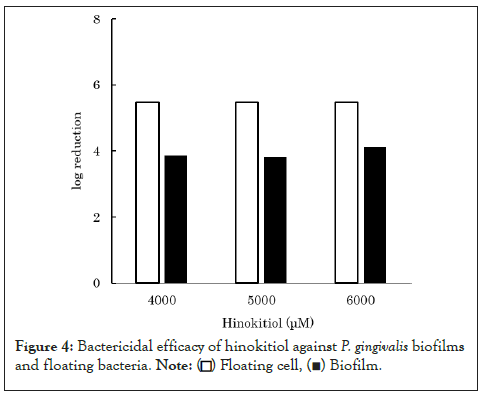
Figure 4: Bactericidal efficacy of hinokitiol against P. gingivalis biofilms and floating bacteria.
Suppression of gingipain production in P. gingivalis by hinokitiol
Studies using the luxS gene-deletion variant (a gene encoding AI-2) revealed that an AI-2-mediated quorum sensing mechanism is involved in the expression of gingipains, a major pathological factor of P. gingivalis [6-8]. Because this study demonstrated hinokitiol’s AI-2 inhibitory activity, we examined the hypothesis that hinokitiol also suppresses the expression of gingipains in P. gingivalis. To clarify the relationship between hinokitiol and gingipain production in P. gingivalis, we performed a western blot using an anti-gingipain antibody (Anti-GP Rabbit Ab, provided by Prof. Kadowaki at Nagasaki University [16] that reacts with a common epitope of Rgp and Kgp. After P. gingivalis was cultured in 30 μM hinokitiol-added medium for 30 minutes, the expression quantities of membrane-binding Rgp and secreting-type Rgp and Kgp from the bacterial bodies were analysed. In the presence of hinokitiol, the membrane-binding Rgp and secreting-type Rgp and Kgp bands were detected at 70-90 kDa, 44 kDa, and 51 kDa, respectively. The expression appeared to decrease, although there were no significant differences (Figures 5a and 5b) [12]. These results suggest that hinokitiol reduces the pathogenicity of P. gingivalis by inhibiting the AI-2 quorum sensing mechanism and suppressing the production of gingipains.
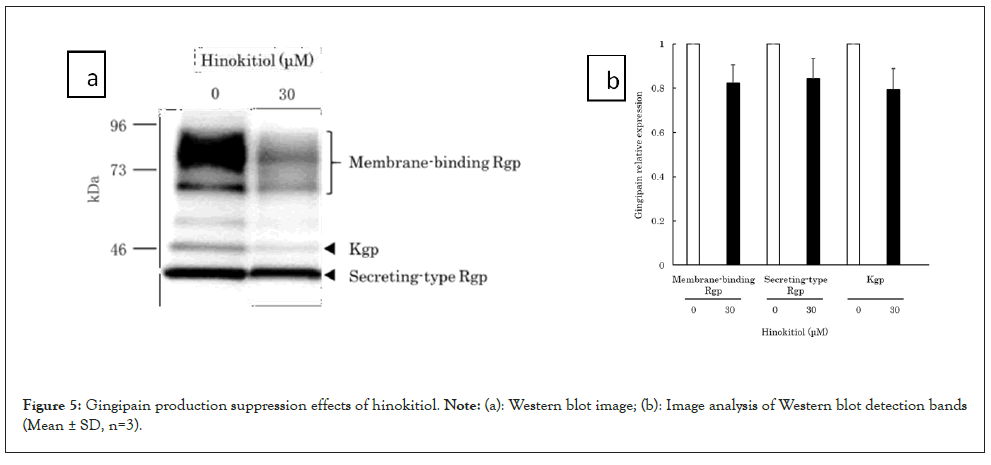
Figure 5: Gingipain production suppression effects of hinokitiol. Note: (a): Western blot image; (b): Image analysis of Western blot detection bands (Mean ± SD, n=3).
In this study, we examined the hypothesis that hinokitiol has the mechanism to act against P. gingivalis by inhibiting AI-2, which is involved in quorum sensing to promote biofilm formation and gingipain production of P. gingivalis. It was demonstrated that in addition to its antiseptic properties, hinokitiol inhibits the AI-2 mediated quorum sensing in P. gingivalis and suppresses biofilm formation and gingipain production, thereby suppressing the incidence of periodontal disease. Hinokitiol exhibited these properties at low concentrations without exerting bactericidal effects on P. gingivalis. Therefore, hinokitiol may be more effective in the treatment of periodontal disease compared to other antiseptics.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Hamada S, Gomi M, Yoshida A (2022) Hinokitiol Acting against Porphyromonas gingivalis; By Inhibiting Autoinducer-2: A Short Communication. Ann Essence Dent. 14: 274.
Received: 29-Nov-2022, Manuscript No. AEDJ-22-20809; Editor assigned: 01-Dec-2022, Pre QC No. AEDJ-22-20809 (PQ); Reviewed: 15-Dec-2022, QC No. AEDJ-22-20809; Revised: 22-Dec-2022, Manuscript No. AEDJ-22-20809 (R); Published: 30-Dec-2022 , DOI: 10.35248/0976-156X.23.14.241
Copyright: © 2022 Hamada S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.