
Advances in Pediatric Research
Open Access
ISSN: 2385-4529

ISSN: 2385-4529
Research Article - (2023)Volume 10, Issue 1
Background: Some children can develop severe forms of SARS-CoV-2 infection either acutely or later, as represented by Multisystemic Inflammatory Syndrome in Children (MIS- C). To identify the risk factors for worse outcomes in hospitalized children and adolescents with severe acute SARS-CoV-2 infection and MIS-C.
Methods: This multicenter cohort study included all children and adolescents with confirmed or suspected critical SARS-CoV-2 infection admitted to the Pediatric Intensive Care Unit (PICU) between April 2020 and September 2021. The exclusion criteria were incomplete vaccinal status, immune-compromised status, and end-of-life decision. The main variables analyzed were epidemiological, clinical, and laboratory data, and ventilator settings at admission and after 72 h. The patients were divided into three groups (G): confirmed coronavirus disease (COVID-19) with MIS-C criteria (G1), confirmed COVID-19 without MIS-C criteria (G2), and MIS-C criteria without confirmed COVID-19.
Results: The median age of the patients was 28 months in G1, with comorbidities in 40 patients (72.7%) (p < 0.0001). The duration of exposure (median 23 days; p = 0.004) and fever were longer in G1 (12 days; p = 0.001). Moreover, Invasive Mechanical Ventilation (IMV) was required in 44 patients (80%, p < 0.0001), and cardiogenic shock occurred in 26 patients (54.2%, p < 0.0001) in G1. Sub-nutrition was most frequent in G1 in 55 cases (57.3%; p = 0.01). Under nutrition (< 2 SD for weight), longer exposure time (odds ratio [OR]: 2.11; 95% confidence interval [CI]: 1.37–3.25; p = 0.001), IMV time (OR: 2.6; 95% CI: 1.15–5.85; p = 0.03), and length of hospital stay (OR: 10.94; 95% CI: 1.93–63.1; p = 0.007) were associated with critical MIS-C in G1.
Conclusion: In the Brazilian Amazon area, specifically in the Pará state, we identified a cluster of more severe forms of pediatric acute or late SARS-CoV-2 infection.
COVID-19; Coronavirus; MIS-C; Multisystemic inflammatory syndrome
The Coronavirus disease (COVID-19) outbreak has predominantly affected the adult population, while children present with relatively mild disease [1,2]. However, some children can develop severe forms of SARS-CoV-2 infection, either acutely or later, as represented by multisystem inflammatory syndrome in children (MIS-C); this is currently considered a rare COVID-19 post-infectious complication, a severe and newly emerging phenotype distinct from COVID-19 infection [3-5].
The aforementioned studies were mostly conducted in economically developed regions, and differences in the infrastructure of health services between these areas can result in distinct patterns of disease progression. Moreover, during the COVID-19 outbreak, some novel serotypes of SARS-CoV-2, causing different clinical scenarios and outcomes, have been identified globally [5-7]. Therefore, the risk factors for worse outcomes of COVID-19 forms in these studies may not be entirely applicable to other geographic regions or to less developed countries, which limits the extrapolation of the results.
To date, there is limited information on the risk factors for pediatric critical COVID-19, acute infection and post-infection, and its outcomes, and only a few case series have been reported in resource-limited settings, such as the Amazon region [8]. The aim of this study was to identify risk factors for worse outcome associated with severe COVID-19 and MIS-C in hospitalized children in a limited care setting, such as the Eastern Brazilian Amazon region.
Study design and participants
Between April 2020 and September 2021, we conducted a multicenter prospective cohort study enrolling all children and adolescents with suspected or confirmed SARS-CoV-2 infection admitted to the Pediatric Intensive Care Unit (PICU) from three reference hospitals in the eastern Amazon area (Brazil): Fundação Santa Casa de Misericórdia do Pará (FSCMPA), Fundação Hospital das Clínicas Gaspar Viana (FHCGV), and Hospital e Pronto Socorro Municipal Mário Pinotti (HPSMMP).
The study was approved by the institutional review board of the coordinating center (the other centers were co-participants). The parents or guardians of the children, or, when applicable, the children themselves, provided written informed consent before being included in the study.
The inclusion criteria were all children and adolescents (1 month to 21 years old) with suspected or confirmed severe SARS-CoV-2 infection or MIS-C who were admitted to the Pediatric Intensive Care Unit (PICU). We excluded patients who were at end-of-life decision stages, immune-compromised patients, and those with unknown or incomplete vaccination status. Incomplete vaccination can lead to greater susceptibility to different agents and, therefore, a greater possibility of serious diseases, especially in children under 5 years of age. In cases of readmission in the PICU, only the first hospitalization was considered.
Case definition
The patients included in the study were divided into three [3] groups (G): G1 - patients with laboratorial evidence of current or previous SARS-CoV-2 infection who fulfilled the World Health Organization (WHO) MIS-C criteria, [9]; G2, patients with laboratorial evidence of current or previous SARS-CoV-2 infection, who did not fulfill the MIS-C criteria; and G3, patients who fulfilled MIS-C criteria without laboratorial evidence of current or previous SARS-CoV-2 infection, or who presented positive epidemiological background for COVID-19 infection but had a proven infection by other agents at admission (this subgroup will serve as a control for groups 1 and 2 regarding outcomes).
Laboratorial evidence of current and previous SARS-CoV-2 infection was based on having a positive Reverse Transcriptase Polymerase Chain Reaction (RT-PCR) test result or a positive serological test (ELISA or immune-chromatography), respectively. Suspected cases were considered as individuals with epidemiological links and clinical or radiological findings compatible with COVID-19, but the absence of molecular or serological tests, or whose results were inconclusive.
The presence of severe illness was determined by the presence of organ dysfunction requiring monitoring in the PICU, diagnosis of Acute Respiratory Distress Syndrome (ARDS), or respiratory failure, in addition to circulatory shock of any kind, encephalopathy, liver or gastrointestinal dysfunction, myocardial dysfunction or heart failure, coagulation disorders, and acute kidney injury [10]. Multiple Organ Dysfunction Syndrome (MODS) was defined as the simultaneous occurrence of three or more organ dysfunctions, according to previously published criteria [11].
Data collection
Before beginning the survey, consultations with specialists were conducted, and educational lectures were given to the participants of the hospital teams. The researchers received prior training on data collection and were “blinded” to the research hypothesis. During the study, the coordinator and the assistant physicians from the units were responsible for validating the collected data and checking for suspicious errors, outliers, or missing values.
The patients included in the study were followed up prospectively from the date of hospital admission until discharge or death, whichever came first. Surviving patients and control subjects were censored on March 31, 2021. The main variables analyzed were epidemiological, clinical, laboratory, and radiological data, as well as ventilator settings at admission to the PICU and after 72h. All parameters were collected and processed at the participating medical centers, and all involved institutions used the same laboratory network for the requested analyses. All tests were performed according to the manufacturer’s protocol.
The image exams and collection of biological samples were performed in the hospital care sector, according to the individualized clinical indications for each patient and the attending physicians’ opinion.
Qualitative real-time RT-PCR assay for SARS-CoV-2 was performed in samples from nasopharyngeal swabs, sputum, or lower respiratory tract aspirates. Serological tests were performed using immunochromatography or ELISA methods, according to medical request; for qualitative serologies (IgG/IgM), kits provided by the public Central Laboratory of the State of Pará (LACEN-PA) were used. An independent collaborating researcher reviewed the data. No genetic sequencing for SARS-CoV-2 was analyzed in this study; therefore, variants were not identified.
Variable and short outcomes
Baseline information (sex, age, weight, medical or surgical admission, definition of severe sepsis and septic shock, length of PICU stay, complex chronic condition [12], mortality and severity scores [13-15], and dates of admission and discharge), clinical manifestations, microbiological and laboratory findings, and outcome (death) were recorded using standardized data collection forms.
In addition to the epidemiological data, we evaluated the clinical course, associated diagnosis of MODS, ventilator settings, arterial blood gas analysis, the outcome, including the need and length of Invasive Mechanical Ventilation (IMV), use of vasoactive drug infusion, weaning failures [16], progression to Ventilator-Associated Pneumonia (VAP) [17], ventilator-free days over a 28-day period [18], development of ARDS [19] and death.
Nutritional status on admission was defined by the Body Mass Index (BMI) Z-score using the most relevant growth standards and references. Z-scores were calculated using the World Health Organization AnthroPlus® software. Subjects were categorized as underweight (BMI Z-score < −2), normal weight (BMI Z-score ≥ −2 and ≤ 1), overweight (BMI Z-score > 1 and ≤ 2), or obese (BMI Z-score > 2) [20].
The primary outcome was the occurrence of severe illness or deaths, while the secondary outcomes were in-hospital mortality at 28 days, length of oxygen supplementation, length of hospital and PICU stay, length of mechanical invasive support, and ventilator-free days at 28 days.
Statistical analyses
Considering that this research is an annual censoring study including all individuals with a diagnosis of COVID-19, no sample size was calculated. Descriptive statistics of continuous variables were reported as the mean and Standard Deviation (SD) for normally distributed data or median and Inter-Quartile Range (IQR) for non-normally distributed data. Categorical variables are described as counts and percentages. Comparisons were assessed using the Kruskal–Wallis test (non-parametric distribution) or Welch’s ANOVA (parametric distribution) for continuous variables and by χ² test or Fisher’s exact test for categorical variables. Adjustments were made for multiple comparisons. Variables with p-values <0.05 in the univariate analysis were included in a multivariate logistic regression model. The level of significance was set at a two-tailed p-value <0.05 with 95% Confidence Interval (CI). All statistical analyses were performed using IBM SPSS Statistics version 25.0.
From April 2020 to September 2021, 528 children and adolescents with suspected or confirmed COVID-19 or MIS-C were admitted to three hospitals. Ultimately, 228 children who required intensive care support were selected for the study and were allocated into three groups (Figure 1).
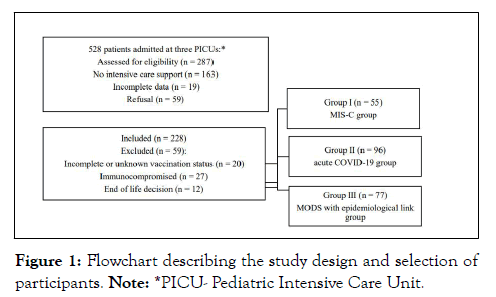
Figure 1: Flowchart describing the study design and selection of participants. Note: *PICU- Pediatric Intensive Care Unit.
The median age of the patients was 28 months in G1 (55 patients), 53 months in G2 (96 patients) and 33 months (77 patients) in G3.
The most used diagnostic method for SARS-CoV-2 infection was the serological test 193 (84.7%), positive in 35 (63.6%) in G1 and 58(60.4%) in G2. Shock Syndrome Kawasaki Disease (SSKD) was the most prevalent clinical form, present in 28 (50.9%) patients from G1. The critical form of SARS-CoV-2 infection was present in 51 (92.7%) patients from G1 and in 48 patients (50%) from G2.
Males were more predominant in G1 than in G2 and G3 (45 [81.8%] vs. 45 [46.9] vs. 31 [40.3]; p = 0.0001). Comorbidity was significantly more common in G1 (40 [72.7%]; p = 0.01). The median duration of exposure and fever were higher in the G1 than in G2 (23 days, p = 0.004, and 12 days, p = 0.001, respectively). According to nutritional assessment, the G2 present more patients classified as underweight (57.3%; p = 0.01) (Table 1).
| Variables | G1 | G2 | G3 | All patients | p-value |
|---|---|---|---|---|---|
| (n = 55) | (n = 96) | (n = 77) | (n = 228) | ||
| Sex - Male, n (%) | 45 (81.8) | 45 (46.9) | 31 (40.3) | 121 (53.1) | < 0.001 |
| Age (months), median (IQR) | 28 (9.2–100.6) | 53 (8.4–90) | 33 (6–84) | 34.7 (6.03–91.6) | 0.545 |
| Clinical admissions, n (%) | 43 (78.2) | 60 (62.5) | 60 (77.9) | 163 (71.5) | < 0.1 |
| Comorbidity, n (%) | 40 (72.7) | 60 (62.5) | 45 (58.4) | 145 (63.6) | 0.01 |
| Exposure form: home, n (%) | 47 (85.5) | 76 (79.2) | 0 (0) | 123 (32.3) | < 0.001 |
| Exposure duration in days, median (IQR) | 23 (15–33) | 6 (5–9) | 0 (0) | 9 (5–16) | 0.004 |
| Fever duration in days, median (IQR) | 12 (9–15) | 4 (2–6.75) | 4.5 (3–7.15) | 2.6 (0–6.5) | 0.001 |
| Nutritional status: underweight, n (%) | 27 (49.1) | 55 (57.3) | 37 (48.1) | 119 (52.2) | 0.01 |
| Productive cough, n (%) | 44 (80) | 65 (67.7) | 45 (58.4) | 154 (67.5) | < 0.001 |
| Severe dyspnea, n (%) | 24 (53.3) | 37 (38.5) | 7(9.1) | 68 (29.8) | < 0.001 |
| Lymphadenomegaly, n (%) | 31 (56.4) | 12 (12.5) | 44 (57.1) | 87 (38.2) | < 0.001 |
| Gastrointestinal symptoms*, n (%) | 48 (87.3) | 62 (64.6) | 17(22.1) | 127 (55.7) | < 0.001 |
| Cutaneous symptoms**, n (%) | 49 (89.1) | 30 (31.3) | 34 (44.2) | 113 (49.6) | < 0.001 |
| Cutaneous rash only, n (%) | 47 (85.5) | 23 (24.0) | 34 (44.2) | 104 (45.6) | < 0.001 |
| Pneumonia at admission, n (%) | 53 (96.4) | 59 (61.5) | 43 (55.8) | 155 (68) | < 0.001 |
| Use of oxygen, n (%) | 55 (100) | 77 (80.2) | 77 (100) | 209 (91.7) | < 0.001 |
| Oxygen therapy time in days, median (IQR) | 8 (6–13) | 3 (2–7) | 4 (2–7) | 3 (2–7) | 0.001 |
| Invasive mechanical ventilation use, n (%) | 44 (80) | 43 (44.8) | 37 (48.1) | 124 (54.4) | < 0.001 |
| IMV in days, median (IQR) | 5 (3–10) | 2 (0–5.25) | 2 (0–6) | 0 (0–5) | 0.001 |
| Ventilator-free days at day 28th, median (IQR) | 0 (0–1) | 1 (0–2.75) | 1 (0–3) | 0 (0–2) | <0.001 |
| VAP, n (%) | 13 (23.6) | 10 (10.4) | 4 (5.2) | 27 (11.8) | < 0.001 |
| ARDS, n (%) | 33 (60) | 32 (33.3) | 25 (32.5) | 90 (39.5) | < 0.001 |
| Severe ARDS, n (%) | 11 (20) | 9 (9.4) | 4 (5.2) | 24 (10.5) | 0.003 |
| Vasoactive drugs use, n (%) | 45 (81.8) | 39 (40.6) | 73 (94.8) | 157 (68.9) | < 0.001 |
| Cardiogenic shock, n (%) | 26 (54.2) | 16 (41.0) | 5 (6.5) | 47 (20.6) | < 0.001 |
| PICU LOS in days, median (IQR) | 7 (5–13.5) | 3 (0–5) | 3 (2–8.25) | 4 (0–7) | < 0.001 |
| Hospital LOS days, median (IQR) | 14 (10–20) | 6.7 (4.5–11) | 15 (10.5–19.5) | 9 (6–15) | < 0.001 |
| Death at 28 days, n (%) | 24 (43.6) | 13(13.5) | 26 (33.7) | 63 (27.6) | < 0.001 |
Note: *Diarrhea, nausea, vomiting, and abdominal pain; **cutaneous rash, other mucosal lesions, oral manifestations. ARDS- Acute Respiratory Distress Syndrome, VAP- Ventilator Associated Pneumonia, PICU- Pediatric Intensive Care Unit, LOS: Length of stay, IMV- Invasive Mechanical Ventilation, IQR- Inter-Quartile Range
Table 1: Epidemiological, clinical, intensive care support, therapeutic management and signs/symptoms of all are included in patients and groups.
The need for IMV and the duration of IMV were significantly higher in G1 than in G2 and G3 (44 [80%], p < 0.0001; 5 days, p = 0.001, respectively). Severe ARDS was also significantly more frequent in G1 than in G2 and G3 (11 [20%] vs. 9 [9.4%] vs. 4 [5.2%]; p = 0.003). Cardiogenic shock was predominant in G1 and G2, with 26 (54.2%) and 16 (41%) cases (p < 0.0001), respectively (Table 1). The overall mortality of the sample was 63 deaths (27.6%), with the highest mortality observed in G1 with 24 (43.6%), (p < 0.0001) (Table 1). G1 patients had a marked median increase in D-dimer levels at admission (G1: 1938.32 ng/dL; p = 0.0014) and at day 3 (G1: 1275.41 ng/dL; p = 0.047). The median CRP/albumin ratio was higher in G1 (day 1: 17.71; p < 0.0001 and day 3: 8.67; p = 0.037). Moderate hypoxemia on admission, assessed by Oxygen Index (OI), was more observed in G1 (median: 8.8; p < 0.0001) (Table 2).
| Variables | G1 | G2 | G3 | All patients (n = 228) | p-value | Reference range |
|---|---|---|---|---|---|---|
| (n = 55) | (n = 96) | (n = 77) | ||||
| Tidal volume (ml/kg) day 1* | 9.9 (1.9) | 7.7 (2.5) | 9.8 (2.3) | 8.1 (2.68) | < 0.001 | - |
| Peep (cmH2O) day 1** | 8 (7–10) | 7 (6-8) | 7 (6–8) | 6 (5–8) | < 0.001 | - |
| Partial pressure of carbon dioxide (pCO2), day 3* | 56.3 (42.4) | 37.6 (13.7) | 35.7 (18.3) | 39.1 (22.1) | < 0.006 | 35–45 mmHg |
| Hemoglobin day 1* | 9.98 (1.36) | 11.7 (1.8) | 16.7 (1.74) | 11.2 (1.84) | < 0.001 | 12–18 g/dL |
| Hemoglobin day 3* | 10.1 (1.4) | 11.8 (1.9) | 10.85 (1.5) | 11.3 (1.8) | < 0.001 | 12–18 g/dL |
| Lymphocytes day 1** | 1249 (963–1756) | 2278 (956-3895) | 2265 (1003–3974) | 1998 (1051–3895) | < 0.001 | 1.500–4.000/mm3 |
| Prothrombin time day 1** | 16 (14.3–17.5) | 15.3 (14.3-17.7) | 13.6 (11.45–21.5) | 14.9 (12.9–17.5) | < 0.001 | 12-14” |
| Prothrombin time day 3** | 15.3 (14.3–17.7) | 14.85 (13.2-17) | 15.3 (14.3–17.5) | 15 (13.8–17.5) | < 0.001 | 12-14” |
| D-Dimer day 1** | 1938.3 (1084.9–5008.5) | 1192.1 (529.33-3336.9) | 892.2 (809.7–944.7) | 769.8 (358–1230) | < 0.001 | < 500 mg/dL |
| D-Dimer Day 3** | 1275.41 (605.51–3700) | 853.81 (417.35-2851.3) | 527.54 (478.46–707.7) | 490.92 (261.72–830) | < 0.001 | < 500 mg/dL |
| International normalized ratio day 3** | 1.41(1.2–1.6) | 1.27 (1.13-1.55) | 1.33 (1.2–1.64) | 1.27 (1.18–1.58) | < 0.001 | 0.8-1.0 |
| C-reactive protein day 1** | 50.1 (22.85–86.1) | 7.6 (1.9-14) | 3.81 (1.41–11.02) | 7.7 (2.3–19.5) | < 0.001 | <6mg/dL |
| Phosphorus day 1* | 2.7 (0.81) | 3.3 (1.15) | 4.6 (1.7) | 4.0 (1.7) | < 0.001 | 2.5–4.5 mg/dL |
| Phosphorus day 3* | 3.4 (1.2) | 4.6 (1.77) | 4.4 (1.51) | 4.4 (1.7) | < 0.001 | 2.5–4.5 mg/dL |
| CRP/albumin ratio day 1** | 17.71 (7.4–28.6) | 2.29 (0.71-6.84) | 2.72 (0.67–5.3) | 2.5 (0.79–7.2) | < 0.001 | - |
| CRP/albumin ratio day 3** | 8.67 (2.94–19.09) | 1.99 (0.52-4.57) | 4.54 (1.87–7.69) | 2.4 (0.66–6.42) | < 0.001 | - |
| Oxygenation index day 1** | 8.8 (4.88–13.18) | 3.55 (1.89-6.01) | 3.51 (2.08–5.82) | 3.72 (2.07–8.02) | < 0.001 | 4–8: mild |
| 8–16: moderate | ||||||
| > 16: severe |
Note: *mean (SD); **median (IQR); Peep: Positive end expiratory pressure. CRP: C-reactive protein.
Table 2: Laboratory, gasometry, and ventilator setting features of all are included in patients and groups.
In general, patients diagnosed with MIS-C were associated with greater need for intensive support and worse clinical outcomes, compared to patients with severe and/or critical COVID-19. Multivariate analysis identified exposure time >21 days and OI at admission up to 8 as independent risk factors for the presence of critical SARS-CoV-2 infection. Prothrombin Time (TP) levels >15s at admission were associated with a higher risk of death, while underweight was associated with longer permanence in the IMV and at the hospital (Table 3).
| Procedure | OR | CI 95% | p–value |
|---|---|---|---|
| Severe or critical clinic form of SARS–CoV–2 infection | |||
| Exposure time more than 21 days | 2.1 | 1.4–3.25 | 0.001 |
| Oxygen index at admission up to 8 | 1.4 | 1.1–1.8 | 0.005 |
| Death | |||
| Prothrombin time in seconds day 1 longer than 15” | 1.4 | 1.1–1.7 | 0.001 |
| Length of stay at PICU up to 7 days* | |||
| Ventilator associated pneumonia | 1.25 | 1.1–1.5 | 0.005 |
| Length of stay at the hospital up to 7 days* | |||
| Male | 10.5 | 1.8–62.4 | 0.01 |
| Under weight | 10.9 | 1.9–63.1 | 0.007 |
| Ventilator–free days at day 28* | |||
| Length of stay in PICU up to 7 days | 1.1 | 1.1–1.2 | 0.026 |
| Ventilator associated pneumonia | 16.3 | 1.2–22.4 | 0.043 |
| Phosphorus <3 mg/dL | 6.1 | 1.1–33.0 | 0.035 |
| Invasive mechanical ventilation time up to five* | |||
| Partial pressure of carbon dioxide (pCO2) up to 45 mmHg on day 3 | 1 | 0.96–1.99 | 0.041 |
| Underweight | 2.6 | 1.15–5.85 | 0.03 |
Note: *These cut-off values are based on the 75th percentile of each outcome, logistic regression forward stepwise procedure. PICU- Pediatric Intensive Care Unit.
Table 3: Multivariate analysis.
This prospective multicenter regional study analyzed the clinical spectrum of SARS-CoV-2 infection in hospitalized children, including acute infections and MIS-C. The aim of this study was to evaluate the factors associated with severe clinical evolution, as well as the short-term clinical outcomes, in the first year of the pandemic, in a scenario of limited resources, and to add new knowledge about the COVID-19 presentation that will be useful in the prevention and control of the eventual third wave of the epidemic. Few previous studies have evaluated the factors associated with poor outcomes in pediatric COVID-19 and MIS-C [21-26].
Several epidemiological and clinical similarities were identified between this study and others [1-3,27-30], with an emphasis on the predominance of males [3,4,30], individuals of mixed race [5,31], clinical admissions [6,32], presence of comorbidities [7,33], pronounced gastrointestinal and skin manifestations [27,29,30], high demand for vasoactive drugs [26,31], as well as the presence of circulatory shock (mainly cardiogenic) [32,33], presence of ground-glass pneumonia [34,35], long hospital stay and PICU, and need for invasive ventilatory support [2-6,36].
It has been hypothesized that the findings described above may be related to a myriad of factors, including genotypic/phenotypic characteristics and unregulated host immune responses, as well as economic and geographical peculiarities, which may limit early access to health services in a health-care limited setting [26,37-41].
Another relevant aspect for the divergences evidenced in countries with low per capita income and/or with peculiar socioeconomic and cultural characteristics is the increased likelihood of neglecting community illnesses. This has a direct and indirect impact on the clinical expression of pathologies, as well as on preventive and curative care strategies. Therefore, studies focused on this epidemiological niche are of great value in the context of deconstructing this reality [42-44].
It is noteworthy that in this study, lymphopenia, OI, SIG and CRP/albumin ratio, and the occurrence of blood disorders were associated with the presence of severe MIS-C. Interestingly, OI, SIG, and CRP/albumin ratio are used to assess hypoxemia, tissue perfusion, and acute phase inflammatory proteins, respectively, while lymphopenia is related to the maturity and functionality of the immune system, both of which can lead to endothelial damage and, consequently, coagulation disorders, all of which determine an imbalance between organic metabolic supply and demand. In addition, an unregulated immune response may be related to unfavorable outcomes.
It is noteworthy that VAP and underweight in this sample were relevant in the independent association with short-term outcomes both in relation to the length of hospital stay and PICU, as well as prolonged duration on IMV and shorter IMV-free days. These findings are not in line with most studies of MIS-C, in which obesity related to the chronic inflammatory process is a hypothetical predisponent to explain the emergence of a cytokine storm [44-48].
In the current study, the overall mortality in patients with MIS-C was higher compared to other studies, and the association of cardiac complications and the need for mechanical ventilation occurred in both groups (G1 and G2), but were more common in post-acute patients, who were also more likely to receive intravenous steroids and immunoglobulins. Other studies reported a similar finding, with the use of human immunoglobulin in 77% of the SIM-P cases, the use of corticosteroids in 49%, and the use of ECMO in 9% of the cases [1-3,27,43,44].
One in ten deaths among children younger than 5 years in low-income and middle-income countries is attributable to severe malnutrition because wasted children are at increased risk of mortality from infectious diseases. Disruptions resulting from the COVID-19 pandemic are expected to continue to exacerbate these scenarios [40,41,43,48]. Therefore, this well-known association between mortality, malnutrition, and greater severity of infectious processes could reinforce the explanation of the high mortality rates associated with the current socioeconomic situation of the pandemic in both waves, which have caused damage to the global community, especially to Brazil, where economic, political, and social strategies aimed at coping with COVID-19 are extremely fragile.
It is also emphasized that in services with better health assistance resources, cardiopulmonary assistance, invasive hemodynamic monitoring such as ECMO, and continuous monitoring of hemodynamic variables was offered; however, such technological resources are not available in our service, and in addition to the conditions of bad accessibility to health services in the Amazon region as well as the previously described economic factors make the prognosis worse [3-6,40,41].
In our cohort, being less than 24 months old, male, with signs or symptoms of lower respiratory tract infection, presence of a pre-existing medical condition, cardiac involvement, and certain abnormal laboratories were associated with a greater likelihood of requiring PICU admission. Moreover, a significant proportion of these patients required ICU support, often including mechanical ventilation with high doses of vasoactive drugs. Our results also show that most children who are intubated due to respiratory failure require prolonged ventilation, usually for one week or more. Coagulation disturbs and underweight are associated with higher mortality and length of stay in the PICU and hospital, respectively.
To the best of our knowledge, this is the first observational study to examine the relative risk of severe COVID-19 and associated mortality among children in Brazil. This study is one of the few in which data from laboratory confirmed patients with COVID-19 were collected prospectively and during admission.
In the Brazilian Amazon, we identified a cluster of more severe forms of MIS-C, and promptly share these results as an urgent public health research priority. Multisystem inflammatory syndrome is a new pediatric disease that is dangerous and potentially lethal. With prompt recognition and medical attention, most children will survive, but the long-term outcomes of this condition are presently unknown.
There are some limitations in our study. First, the limited sample size; second, children were classified using a severity classification that has been previously applied to other pediatric cohorts, which is designed primarily for respiratory symptoms and lung involvement; and finally, the population is based on hospitalized children, which can lead to more severe forms of SARS-CoV-2 infection and may explain the higher frequency of viral pneumonia among the severe phenotypes, but not among patients requiring ICU admission. Moreover, multiple variants of the virus are recognized; however, as viral genotypic determination was not performed in this study, it would be speculative to suggest such cause and effect at this point.
Therefore, in the present study, the subset of critical patients not only included patients with respiratory failure, but also with other potentially fatal conditions. Consequently, in addition to reporting the clinical characteristics, risk factors, and outcomes of COVID-19 in children, this dataset provides a unique opportunity to objectively monitor the emergence and progression of another kind of multisystemic inflammatory syndrome in Brazil, which was the center of the second wave, while minimizing memory bias.
We declare this is our original work. We do confirm that the manuscript is original, has not already been published in a journal and is not currently under consideration by another journal.
We have got Ethical approval from Ethical Review Board of Fundação Santa Casa do Pará Belém, Pará, Brazil (Protocol No: APHIHIRTD/343/2019). Assent was obtained from children and written informed consent was obtained from caregivers. Besides, confidentiality was maintained by keeping privacy at all levels of the study. The study was conducted in accordance with the Declaration of Helsinki.
First of all, we are glad to thank Fundação Santa Casa de Misericórdia do Pará for giving us chance to conduct this research. Our special thanks go to all the children, parents, and participated in PICU.
All the datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Funding was not received from any organization.
Not Applicable.
We declare no competing interests.
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
Citation: Carlos Franco de Farias E, Pavão MJC, de Sales SCD, do Nascimento LMPP, Pavão DCA, dos Santos VTS, et al. (2023) Hospitalized Children with Critical SARS-CoV-2 Infection Cared for in a Limited Resource Setting: Multicenter Cohort Study. Adv Pediatr Res. 10:052.
Received: 17-Jan-2023, Manuscript No. LDAPR-23-21410; Editor assigned: 19-Jan-2023, Pre QC No. LDAPR-23-21410 (PQ); Reviewed: 03-Feb-2023, QC No. LDAPR-23-21410; Revised: 13-Feb-2023, Manuscript No. LDAPR-23-21410 (R); Published: 21-Feb-2023 , DOI: 10.35248/2385-4529.23.10.052
Copyright: © 2023 Carlos Franco de Farias E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.