
Advances in Pediatric Research
Open Access
ISSN: 2385-4529

ISSN: 2385-4529
Research Article - (2024)Volume 11, Issue 1
Background: Neonatal hypoglycemia is a frequent problem with potential neurological adverse effects and it involves a significant number of admissions to neonatal units with the consequent mother-child separation, difficulty in establishing breastfeeding and increased hospital expenditure. Although any newborn can suffer hypoglycemia, certain patients are at higher risk. Strategies to detect and prevent hypoglycemia in this subgroup of patients is a common practice in neonatology units. This study aims to analyze the impact of this situation in our center, in order to identify, areas for improvement.
Material and methods: We performed a retrospective review of newborns admitted for hypoglycemia in a tertiary hospitalization center during 2019 and 2020.
Results: 232 patients were admitted for hypoglycemia during this period, accounting for 11.5% of total admissions. Of these, 185 (79%) had known risk factors for hypoglycemia. The median gestational age was 37 (Interquartile Range (IQR) 36-38) and weight 2450 g (IQR 2255-2935 g). The most frequent risk factor was late prematurity (30.8%), followed by low birth weight (26%). Ninety percent had a pathological first blood glucose level. Median chronological age at admission was 6 (Resident Intelligence Quotient (RIQ) 4-10). A total of 42.7% were exclusively Breastfed (BF), 31.3% fed with Adapted Formula (AF) and 16.75% of the patients had not taken early enteral feedings (neither formula bottle nor breastfeeding) before the first glycemic control. The mean of the first glycemic value was significantly lower in those who did not take an early feed (mean 28.4, Standard Deviation (SD) 1.7) than those who did (mean 35.2, SD 0.88).
Conclusion: Admissions for neonatal hypoglycemia are frequent. Early intake was associated with a higher first glycemic control. This measure was only fulfilled within some patients in our center, so there is an opportunity for improvement.
Newborn; Hypoglycemia; Breastfeeding; Improvement
Neonatal hypoglycemia is a common problem with potential adverse neurological effects. It causes significant admissions to neonatal units with the consequent mother-child separation, difficulty establishing breastfeeding and increased hospital costs [1-5].
The fetus receives a constant supply of glucose through the placenta, which is interrupted at birth. All newborns present a physiological drop in blood glucose of up to 30 mg/dL (mean blood glucose 55 mg/dL) that is mild, transient (first two hours of life) and without consequences in the vast majority of cases. During this period, hepatic glycogen deposits are stimulated and insulin decreases, allowing regulation of hydrocarbon metabolism until stability is achieved around 18-36 hours of life [6-10].
Some newborns, either due to a deficit in glycogen stores (premature and low birth weight), increased needs (high birth weight) or transient hyperinsulinemia (children of mothers with carbohydrate intolerance), have more difficulty in self-regulating blood glucose levels. These are what we consider newborns at risk of early transient hypoglycemia [11]. For all these reasons, hospitals have implemented measures to detect and prevent this situation [12-15].
The definition of hypoglycemia is controversial and various cut- off points have been proposed depending on the type of patient and age. Recent studies found that adrenergic responses and increased cerebral flow occurred with glucose concentrations below 47 mg/dL (2.6 mmol/L), although these infants had no clinical signs of hypoglycemia. It has also been seen that if a level >47 mg/ml is maintained, there are no adverse effects, so a lower value has been defined as hypoglycemia [13,16-18].
The most frequently implemented preventive measures are early initiation of enteral feeding (first hour of life), frequent feedings (every two hours), reinforcement with adapted formula or expressed breastfeeding if available and in some centers, 40% concentrated oral dextrose gels are used [9,11,17,18].
The clinical manifestations of neonatal hypoglycemia are not specific. Its severity is highly variable and includes changes in the level of consciousness, lethargy, stupor with slight hypotonia, tremors, poor sucking and vomiting. And in more severe forms irregular breathing, tachypnea, cyanosis and convulsions.
In our center, in patients at risk, the administration of an early feeding is indicated, which is always breastfeeding unless there is a specific parental choice or inability to initiate early breastfeeding (due to maternal situation, difficulties in latching, hypoagalactia). In these cases, artificial formula is offered. After the early feeding, a first blood glucose control is indicated before the next feeding. If the patient has two controls above 47 mg/dL, it is considered that the patient does not require further specific monitoring (as long as there are no symptoms of hypoglycemia or doubts about the effectiveness of the feeding). If any value is pathological, it is indicated to perform a feeding (breast or artificial formula) and to conduct a new subsequent control. If it is pathological again or the patient presents symptoms, admission to the unit and start intravenous glucose administration is indicated.
If at any time a patient presents a control <25 mg/dL and or symptoms of hypoglycemia, it is considered severe or symptomatic hypoglycemia, respectively and requires admission at that time, administration of a bolus of glucose at 200 mg/kg and initiation of intravenous glucose fluid therapy [19].
Despite all the control and prevention measures, these patients continue to be admitted to neonatal units and the number of susceptible patients is increasing (due to the increase in prematurity or diabetic maternal pathology) [20,21].
We proposed a study to analyze the magnitude of this clinical situation in our center and whether there was a possibility of implementing improvement measures to reduce admissions and mother-child separation for this reason.
A retrospective study of all term or near-term neonatal patients admitted for transient early hypoglycemia during 2019-2020 in a level IIIC neonatal unit was performed.
Primary outcome
To analyze the characteristics of those patients who required admission to our unit for early hypoglycemia and the treatment applied to them to evaluate possible improvement actions.
Secondary outcome
• To analyze the clinical course (minimum glycemia, presence of symptoms, need for glucose bolus, time of serum therapy and admission) between patients according to whether or not they have taken an early feeding and the type of feed.
• To analyze the clinical course (minimum glycemia, presence of symptoms, need for glucose bolus, time of serum therapy and admission) according to the patient's risk factor: low or high weight for gestational age, children born to mothers with diabetic pathology or late prematurity.
Inclusion criteria
Newborns admitted at term and late preterm due to early hypoglycemia (as the only reason) during 2019-2020 in our center.
Exclusion criteria
Those who do not meet the inclusion criteria. Those admitted for hypoglycemia but without risk factors for hypoglycemia.
Technical aspects
Glucose measurements are made from heel stick blood and/or venous extraction using Gem4000® point-of-care-testing micro sampling equipment.
Definitions
Neonatal hypoglycemia: Blood glucose value of a capillary sample obtained and properly processed is less than 47 mg/dL using a micro-sample analysis kit (Gem4000®).
Early neonatal hypoglycemia: Blood glucose value below 47 mg/dL detected from birth to the first 48 hours of life.
Severe hypoglycemia: Serum glucose <25 mg/dL.
Symptoms of hypoglycemia: Tremors, irritability, excessive crying, decay, lethargy, vomiting.
Nutritional supplementation: Any dietary intake other than self-breastfeeding given to an infant less than six months of age.
Low weight for gestational age: Percentile <10 for gestational age and sex according to INTERGROWTH-21st charts.
High weight for gestational age: Percentile >90th percentile for age and sex according to INTERGROWTH-21 charts.
Early feeding: Breastfeeding, expressed breastfeeding or adapted formula given in the first two hours of life and before the first glucose control.
Retrospective and cross-sectional analysis
A cross-sectional analysis during one month (December 2021) was conducted analyzing all newborns in our center and collecting data on whether they had a risk factor for hypoglycemia or not, if they did, what the risk factor was and whether they were admitted for this reason.
Statistical analysis
A normality analysis of the continuous variables was performed with the Kolmogorov-Smirnov test. The results of the quantitative variables were expressed by their mean and standard deviation in the case of normal distribution and by their median and interquartile range in the rest of the cases. The results were expressed by their absolute and relative frequencies for qualitative variables. Qualitative variables were compared using the chi-square or fisher's exact test for observed frequencies of less than 5. For the analysis of quantitative variables, the t-student test (parametric variables), Mann Whitney U test (nonparametric variables) and Kruskal Wallis test in the case of non-dichotomous variables were used.
The analytical and statistical analysis was performed using Statistical Package for the Social Sciences (SPSS) (version 21.0). Statistical significance was p<0.05.
The sample size was calculated taking into account the crosssectional results to establish how many newborns at risk of hypoglycemia were admitted for this reason (expected proportion of 6%), a confidence interval of 95%, a precision of 4% and a sample size of at least 135 patients was calculated.
Characteristics of the sample
The unit admitted 2,050 patients during 2019-2020, of which 232 were for hypoglycemia, accounting for 11.3% of total admissions. Of these, 185 (79.7%) had known risk factors for hypoglycemia, with the remaining cases being incidental findings made in patients without risk factors who presented symptoms or poor intake or as accidental findings after a gasometry was performed for altered cord Potential of Hydrogen (pH).
The analysis was performed with the 185 patients who did present known risk factors and therefore fell within the center's hypoglycemia risk management protocol.
The median gestational age was 37 weeks (interquartile range RIQ 36-38) and the median weight was 2450 g (RIQ 2255-2935 g). The median maternal age was 34 years (RIQ 18-46) and they had no history of interest 74% (137/185).
Thirty-five women were diagnosed with gestational diabetes (18.5%), fifteen required insulin for their control and the rest were controlled just with dietary indications. Nine women (4.8%) had pregestational diabetes (6 required insulin). Table 1 shows the essential characteristics such as sex, Gestational Age (GA), anthropometry and perinatal history.
| Variable | Median (IQR)/Absolute value (proportion) |
|---|---|
| Gestational age (weeks) | 37 (36-38) |
| Female sex | 77/185 (41,6 %) |
| Weight (grams) | 2450 (2255-2935) |
| Weight percentile* | 20 (6-68,5) |
| Length (cm) | 47 (45-49) |
| Length percentile* | 28,5 (8,75-70,50) |
| Head circumference (cm) | 33 (32-34) |
| Head circumference percentile* | 35 (17-56) |
| Maternal age (years) | 34 (31-38) |
| Type of delivery | Vaginal delivery 117 (63%) |
| Delivery room resuscitation | No resuscitation 140 (75,6%) |
Note: *: Percentiles according to INTERGROWTH-21st curves; IQR: Interquartile Range.
Table 1: Fundamental characteristics of patients considered to perform the statistical analysis.
Blood glucose controls and admission characteristics
Up to 90% presented an altered first blood glucose value (<47 mg/dl), the median minimum blood glucose value was 31 mg/ dL (IQR 25-36) at 3 hours of life (IQR 2-5) and the median number of hours of life at admission was 6 hours (IQR 4-10).
All of them received supplementation with adapted formula during admission. The vast majority received intravenous glucose therapy (96%), although only 14.5% (27/185) presented symptoms of hypoglycemia. They received intravenous glucose therapy for a median of 30 hours (IQR 7-24) with maximum glucose requirement of 4 mg/kg/min (IQR 2.2-12) and the length of admission was two days (IQR 1-20). More prolonged admissions were inversely related to gestational age (Probability (p)=0.028, Post Cibum (PC)=-0.162), birth weight, length and head circumference (p=0.005, PC=-0.204; p=0.001, PC=-0.248; p=0.000, PC=-0.2,82). It was also significantly related to the first, second and third glycemic values (Respectively: p=0.02, PC=-0.172; p=0.016, PC=-0.194; p=0.011, PC=-0.239) with the minimum glycemic value (p=0.01, PC=-0.248).
Feeding and administration of early feeding
Regarding the feeding received, 86.2% (144/167) of the mothers reported a preference for breastfeeding and only 11.2% (20/167) for adapted formula feeding.
At birth, 42.7% of the newborns (79/185) received early exclusive breastfeeding, 31.3% (58/185) adapted formula and 16.75% (31/185) did not receive adequate feeding before the first glycemic control. In 17 (9.1%) patients, it was not possible to determine whether or not they received an early feeding and what type it was. Some reasons for not receiving early feedings were ineffective latching on to the breast, newborns with early symptoms of hypoglycemia and cesarean delivery with maternal indisposition for early feedings associated with the maternal desire to breastfeed.
Patients who did not receive early feeding were more frequently born by cesarean section (33.5% vs. 16.7%, p=0.029) and presented more frequently the first pathological glycemic value (100% vs. 87.6%, p=0.043); this first value was lower compared to patients who did receive an early feed (mean 28.4, SD vs. mean 35.2, SD 0.88, p=0.001), required earlier admission (mean 5.9 hours of life SD 0.4 vs. 8 hours SD 0.56, p=0.035) and more frequent glucose bolus (26.6% vs. 11.5%, p=0.032). There were no differences between the two groups (early intake or not) in the rest of the factors analyzed, as shown in Table 2.
| Variables | Early feed | |
|---|---|---|
| Performs early feed (138) | Does not perform early feed (30) | |
| Gestational age (weeks) | 37 | 37 |
| Female sex | 46,3% (64/138) | 30% (9/30) |
| Weight (grams) | 2644 ± 54,1 | 2771 ± 150,28 |
| Maternal age (years) | 33,5 ± 0,47 | 34,1 ± 1,2 |
| Vaginal delivery* | 83,3% (115/138) | 66,6% (20/30) |
| Cord blood glucose (mg/dl) | 63 ± 2,2 | 62,1 ± 5,1 |
| First serum glucose (mg/dl)* | 35,2 ± 0,88 | 28,4 ± 1,7 |
| The first glucose value is pathological* (<47 mg/dl) | 87,6% (121/138) | 100% (30/30) |
| Minimum glycemia* (mg/dl) | 30,63 ± 0,60 | 27,36 ± 1,52 |
| Symptoms | 13% (18/138) | 23,3% (7/30) |
| Glucose bolus | 11,5% (16/138) | 26.6% (8/30) |
| Intravenous glucose* | 94,9% (131/138) | 100% |
| Hours of life at admission* | 8 ± 0,56 | 5,9 ± 0,4 |
| Hours of intravenous therapy | 39,4 ± 2,73 | 37,1 ± 6,38 |
| Maximum glucose requirement (mg/kg/min) | 4,57 ± 1,37 | 4,4 ± 1 |
| Days of admission | 3,4 ± 0,24 | 3,6 ± 0,41 |
Note: *: Variables with statistical significance, p<0.05.
Table 2: Comparison of the different variables between the group of patients who did and those who did not take an early feed.
Children who received early breastfeeding were admitted later (mean 9.1 hours SD 0.56 vs. 6.55 SD 0.52, p=0.004) and were more frequently born vaginally (94.4% of those who received BF were born vaginally vs. 69% of those who received AF, p=0.000), as shown in Table 3.
| Variables | Early feed | |
|---|---|---|
| Breast feed (79) | Artificial formula (59) | |
| Gestational age (weeks) | 37 | 37 |
| Female sex | 44,3% (35/79) | 49,1% (29/59) |
| Weight (grams) | 2717,78 ± 66,29 | 2545,42 ± 89,56 |
| Maternal age (years) | 32,7 ± 0,61 | 34,54 ± 0,73 |
| Vaginal delivery* | 94,9% (75/79) | 69% (41/59) |
| Cord blood glucose (mg/dl) | 64,3 ± 2,7 | 60,8 ± 3,9 |
| First serum glucose (mg/dl)* | 35,5 ± 1,1 | 34,74 ± 1,3 |
| The first glucose value is pathological* (<47 mg/dl) | 12,6 % (10/79) | 11,8% (7/59) |
| Minimum glycemia* (mg/dl) | 30,9 ± 0,73 | 30,28 ± 1 |
| Symptoms | 15,1% (12/79) | 10,1 %(6/59) |
| Glucose bolus | 11,3 %(9/79) | 11,8 % (7/59) |
| Intravenous glucose | 92,4% (73/79) | 98,3% (58/59) |
| Hours of life at admission* | 9,1 ± 0,65 | 6,55 ± 0,52 |
| Hours of intravenous therapy | 37,2 ± 3,03 | 42,86 ± 4,85 |
| Maximum glucose requirement (mg/kg/min) | 4,4 ± 0,13 | 4,7 ± 0,2 |
| Days of admission | 3,3 ± 0,32 | 3,6 ± 0,38 |
Note: *: Variables with statistical significance, p<0.05.
Table 3: Different variables' comparisons between patients who relied on early breastfeeding and those who received artificial formula.
Hypoglycemia risk factors
The risk factors for hypoglycemia presented by the newborns were (in decreasing order): prematurity 29.1% (54/185), low birth weight 28.6% (53/185), two factors 21.6% (40/185), high birth weight 9.1% (13/185), a mother with diabetes requiring insulin 6.4% (12/185), a mother with diabetes without insulin 2.7% (5/185) and coincidence of 3 factors 2.1% (4/185). Of those with more than one factor (44 patients in total), 33 were preterm (75%) and 25 were underweight (56.8%).
No significant differences were found in the different risk groups regarding glycemia values, minimum glycemia (Figure 1), having or not having symptoms, serum therapy time or days of admission, as shown in Table 4.
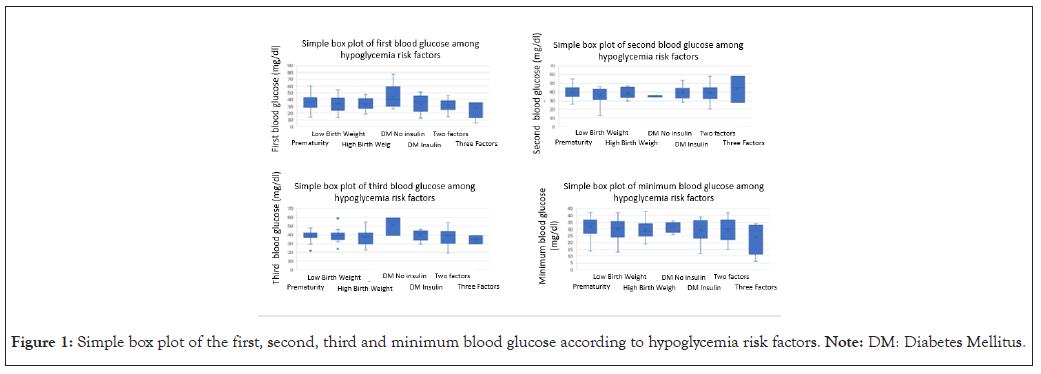
Figure 1: Simple box plot of the first, second, third and minimum blood glucose according to hypoglycemia risk factors. Note: DM: Diabetes Mellitus.
| Variable | Hypoglycemia risk factors | ||||||
|---|---|---|---|---|---|---|---|
| Prematurity | Low birth weight | High birth weight | DM no insulin | DM insulin | Two factors | Three factors | |
| New born | 54 | 53 | 17 | 5 | 12 | 40 | 4 |
| Hours of life when minimum glucose level: Median (IQR) | 3 (2-6) | 3 (2-5) | 4 (2-5) | 4 (2-9) | 3 (2-6) | 3 (2.5) | 4 (1-7) |
| Hours of life at admission: Median (IQR) | 6 (4-12) | 6 (5-8) | 6 (5-10) | 10 (4-12) | 7 (6-10) | 6 (4-10) | 8 (3-10) |
| Symptoms (%) | 14,5% (8/55) | 18% (9/50) | 0% (0/13) | 20% (1/5) | 8% (1/12) | 18,4% (7/38) | 20% (1/5) |
| Hours of intravenous therapy: Median (IQR) | 30 (24-39) | 27 (24-48) | 30 (24-38) | 24 (16-50) | 26 (19-36) | 36 (24-48) | 48 (27-96) |
| Days of admission: Median (IQR) | 3 (2-5) | 2 (2-5) | 2 (2-4) | 1 (1-4) | 2 (2-2) | 2 (1-5) | 3 (2-7) |
Note: DM: Diabetes Mellitus; IQR: Interquartile Range.
Table 4: Clinical outcome variables between the hypoglycemia risk groups.
Cross section
In December 2021, 376 infants were born at the center, of which 83 (22%) had risk factors for hypoglycemia, 19 (5%) had early hypoglycemia and 5 (1%) had to be admitted for this reason. During that month, there were 45 admissions to the unit. Admissions for hypoglycemia accounted for 11% of total admissions.
Newborns with risk factors for hypoglycemia were low weight for gestational age in 30% of cases (25/83), preterm in 19.2% (16/83), children of mothers with non-insulin-dependent diabetes mellitus in 18% (15/83) and children of insulindependent diabetic mothers in 2.4% of cases (2/83). Four patients presented two risk factors (4.8%).
The incidence of hypoglycemia occurred in late preterm (43.7%, 7/16), low birth weight (24%, 6/25), two-factor (25%, 1/4), DM no insulin (20%, 3/15), high birth weight (9,5%, 2/19) and 0 in the group of DM treated with insulin (only 2 patients).
In this sample, late preterm and low birth weight patients were the most frequent risk factors with the highest risk of developing hypoglycemia.
In the present study, we observed a high frequency of admissions for hypoglycemia, in agreement with the literature, representing a significant healthcare expense and an undeniable impact on establishing maternal-filial separation bonding. Compliance with preventive measures for the occurrence of hypoglycemia in at-risk groups was irregular, revealing areas for improvement in managing this pathology in our center.
Admissions for hypoglycemia accounted for a high percentage of total admissions and the great majority had known risk factors for hypoglycemia and in whom, according to protocol, a series of preventive and follow-up measures are prescribed according to international consensus [9,22].
There were significant differences in the first glycemic value and the time of admission, with lower levels and earlier admission for those who did not take an early feed compared to those who did, whether they took adapted formula or breastfeeding. It is also noteworthy that, all those who did not take an early feed, had an altered first blood glucose value.
It has been shown that early breastfeeding in the delivery room improves glucose levels in children born to diabetic mothers and also reduces the incidence of hypoglycemia in the following 8 hours; it is also known that oral stimulation by suction affects intestinal hormones such as gastrin and cholecystokinin, which help regulate insulin secretion [23-25].
Artificial formula has been associated with undesirable effects such as interruption of breastfeeding, increased risk of allergies and infections and alteration in the microbiome. However, some studies also show that it increases glycemia to a greater extent (than breastfeeding) after an episode of hypoglycemia [26]. Its occasional use in patients with significant weight loss increases the breastfeeding rate at one week and three months of life, making it a valid option for preventing and treating hypoglycemia [15,27].
In our sample, between the two early feeding options, the most relevant difference found was the age at admission, which was significantly in those who received breast milk, supporting the previous bibliography of human milk capacity to stabilize glycemia in a more sustained manner [24].
Some reasons for not performing an early feeding were ineffective latching on to the breast, newborns with early symptoms of hypoglycemia (first two hours of life) and cesarean delivery with maternal indisposition for early feeding associated with a maternal desire for breastfeeding. No literature has been found that specifically explores the causes of delay in early feedings. However, it has been found that initiating breastfeeding as late as two hours after delivery have a negative impact on the establishment of effective breastfeeding, which could also hinder the establishment of adequate feedings during admission to the maternity ward [28].
We believe it is essential to facilitate breastfeeding, even if the delivery is by cesarean section and if this is not possible, administer artificial formula in these at-risk patients.
Regarding the risk factors for hypoglycemia, we found that prematurity and low birth weight were the most frequent causes of those admitted for hypoglycemia, not only because they were the most prevalent risk factor but also because the incidence of hypoglycemia was higher in this population. However, once they were admitted, they did not require more days of hospitalization or more glucose intake than those with other risk factors.
Most patients who had to be admitted presented an altered first blood glucose value and it was more frequent that they were admitted in the first six hours of life. This reinforces the importance of stabilization during the first hours by noninvasive means, such as establishing an adequate and early enteral intake.
Admissions for neonatal hypoglycemia are frequent, accounting for 11.5% of all admissions to our unit. Seventy-nine percent of the patients had a known risk factor for hypoglycemia. The factor most associated with admission for this reason was presenting a pathological first glycemic control, a situation observed in 90% of the patients. An appropriate measure to avoid this would be to provide an early feed before the first glycemic control. The mean of the first glycemic value was significantly lower in those who did not perform an early feeding (mean 28.4, standard deviation 1.7) than in those who did (mean 35.2, standard deviation 0.88). Of the patients in our sample, 16.5% did not perform a blood glucose reading prior to glucose measurement.
This measurement was pathological in all of them. We should make an effort to insist on the importance of this early measurement to avoid unnecessary treatment and hospitalization in these patients.
This is a retrospective study with the consequent limitation regarding the loss of relevant data (data from newborns at risk of hypoglycemia that did not require admission).
Likewise, it is a study carried out in one center, revealing a clinical practice that may not be extrapolated to other hospitals; however, since it is a center of the highest level of care with a non-negligible volume of annual deliveries (around 5000/year), it does yield data from a large sample that may reflect the reality of other hospitals of a similar level.
All procedures performed in studies involving human participants adhered to the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study protocol was approved by the ethics committee of the Gregorio Marañón Hospital on 10/01/2023 and granted a waiver of consent.
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
CS collected and analyzed the data and wrote the manuscript. IM designed the study, analyzed the data, and revised the manuscript. MSL designed and reviewed the study.
The authors are grateful for the work of all the personnel involved in managing and treating newborns and their mothers in our unit, in the delivery room, and in the maternity ward.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Delgado CS, Ros IM, Luna MS (2024) Neonatal Hypoglycemia: What Can We Improve? Adv Pediatr Res. 11:074.
Received: 17-Jan-2024, Manuscript No. LDAPR-24-29208; Editor assigned: 19-Jan-2024, Pre QC No. LDAPR-24-29208 (PQ); Reviewed: 09-Feb-2024, QC No. LDAPR-24-29208; Revised: 16-Feb-2024, Manuscript No. LDAPR-24-29208 (R); Published: 23-Feb-2024 , DOI: 10.35248/2385-4529.23.11.074
Copyright: © 2024 Delgado CS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.