
Advances in Pediatric Research
Open Access
ISSN: 2385-4529

ISSN: 2385-4529
Research Article - (2023)Volume 10, Issue 4
Background: Fetal Alcohol Spectrum Disorder (FASD) screening, diagnosis, intervention, research and prevention hinges on establishment of interdisciplinary FASD diagnostic clinics using an evidence-based method of diagnosis. In 1993, Washington State opened the first interdisciplinary FASD diagnostic clinic sponsored by the CDC as a FASD primary prevention study. Clinic data was used to develop the evidence-based FASD 4-digit diagnostic code, paving the way for the clinic’s expansion into a Statewide network of FASD diagnostic clinics (Washington Fetal Alcohol Syndrome Diagnostic & Prevention Network), now in its 30th year. Alaska adopted this Washington model in 1999. Both States have also participated in the CDC Pregnancy Risk Assessment Monitoring System and Behavioral Risk Factor Surveillance System since the 1990s. Study objectives were to describe the two Statewide FASD diagnostic networks; graphically compare the 4-digit-code FASD diagnoses and Prenatal Alcohol Exposure (PAE) over 2-3 decades and illustrate how network data helped guide FASD public health policies and track successful prevention efforts.
Methods: Retrospective descriptive study.
Results: FASD diagnostic outcomes were similar across 2,532 Washington and 2,469 Alaskan patients. PAE in each State followed similar annual trajectories from 1991-2020. Both States documented significant decreases in FAS and PAE in the 1990s. Clinic data helped guide public health policies.
Conclusions: Both States demonstrated the feasibility and value of establishing Statewide interdisciplinary FASD diagnostic clinical networks using the FASD 4-digit-code. Legislative support, centralized data collection and use of a single, evidence-based FASD diagnostic system have been key to the long-term, ongoing success of these two diagnostic networks.
Fetal alcohol spectrum disorder; FASD 4-digit diagnostic code; Statewide interdisciplinary clinical networks; Prenatal alcohol exposure; PRAMS/BRFSS
Alc: Alcohol; CNS: Central Nervous System; H: Height; SD: Standard Deviation; W: Weight; %: Percentile; SDs: Standard Deviations; CDC: Center for Disease Control; FAS: Fetal Alcohol Syndrome; FASDPN: Fetal Alcohol Syndrome Diagnostic & Prevention Network; WA: Washington; AK: Alaska; PAE: Prenatal Alcohol Exposure; PRAMS: Pregnancy Risk Assessment Monitoring System; IEP: Individualized Education Program; SE: Static Encephalopathy; AE: Alcohol-Exposed; ND: Neurobehavioral Disorder; DHSS: Department of Health and Social Services; BRFSS: Behavioral Risk Factors Surveillance System; PFAS: Prenatal Fetal Alcohol Syndrome
Fetal Alcohol Spectrum Disorder (FASD) is a spectrum of physical and neurodevelopmental abnormalities caused by prenatal alcohol exposure [1]. Meaningful progress in FASD screening, diagnosis, intervention, surveillance, public health policy, research and ultimately prevention hinges on establishment of interdisciplinary FASD diagnostic clinics using a single evidence-based, comprehensive and reproducible method of diagnosis of diagnosis [2-4]. In 1993, the University of Washington in Seattle Washington (WA) opened the first interdisciplinary FASD diagnostic clinic sponsored by the Center for Disease Control (CDC) as a FASD primary prevention study [2,3,5,6]. Data collected in the clinic was used to develop and validate the FASD 4-digit diagnostic code in 1997 [4,7-9]. Creation of the diagnostic system paved the way for legislative expansion of the clinic through Senate Bill 5688 into a statewide network of FASD diagnostic clinics, the WA Fetal Alcohol Syndrome Diagnostic & Prevention Network (FASDPN) [10,11]. The WA FASDPN is now in its 30th year of operation. This interdisciplinary Statewide FASD diagnostic system and model was adopted by Alaska (AK) in 1999 and continues to this date [12-14]. The WA interdisciplinary model and 4-digit code have been adopted worldwide. Over 1,700 clinicians from 35 countries have completed the FASD 4-digit diagnostic code online course [15,16]. The primary objective of this study was to demonstrate the feasibility and value of establishing long term, Statewide interdisciplinary FASD diagnostic clinics using the FASD 4-digit diagnostic code. To meet this objective:
1. The WA and AK statewide FASD diagnostic network models are described.
2. The 4-digit code FASD diagnostic outcomes and CDC Pregnancy Risk Assessment Monitoring System and CDC Behavioral Risk Factor Surveillance System prenatal alcohol exposure histories documented over 2-3 decades are graphically summarized [17-21].
3. The impact data from these networks have had on helping: A) Guide FASD public health policy and B) Track successful prevention efforts is illustrated.
Subjects
The diagnostic outcomes of all individuals (birth through adult) with confirmed prenatal alcohol exposure at any level, evaluated by the WA FASDPN from 1993-2021 (n=2,532) and the AK Department of Health and Social Services (now known as the Department of Health) FASD diagnostic teams from 1999-2021 (n=2,469) are graphically compared in this study. These diagnostic outcomes include 4-digit code diagnostic categories A-C, E-J as defined in Figure 1. All data was collected with Institutional Human Subjects approval, shown in Table 1.
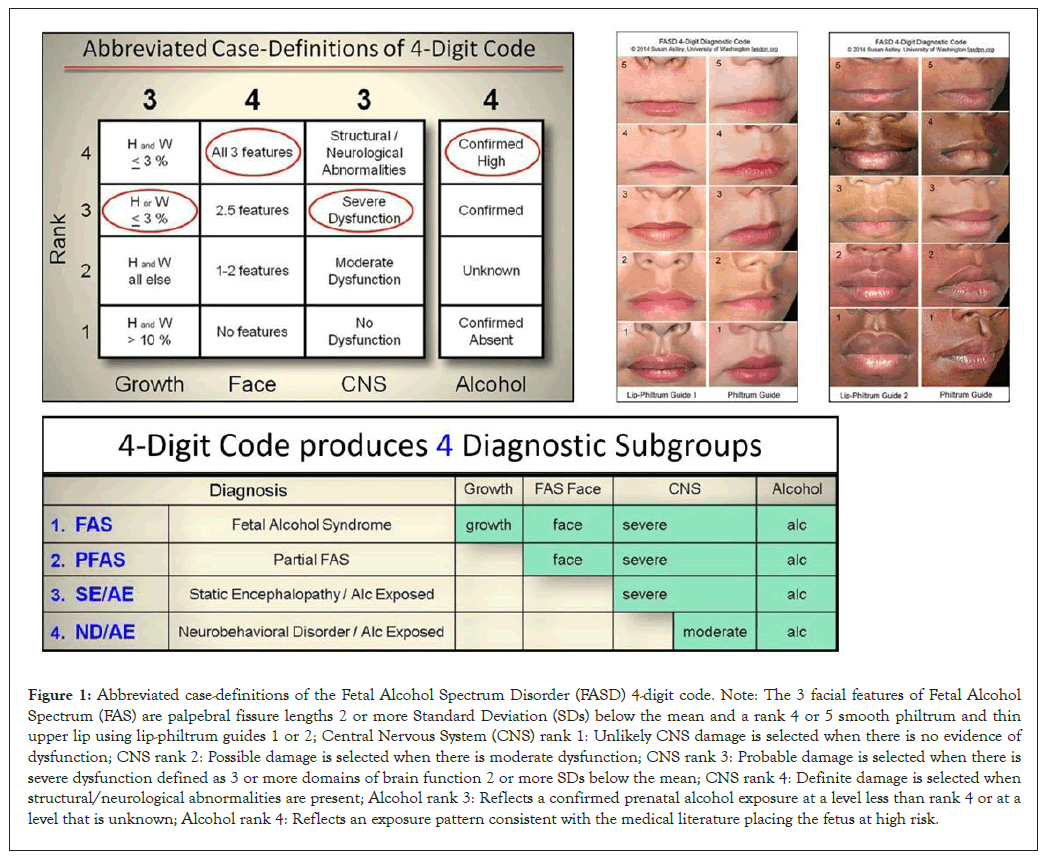
Figure 1: Abbreviated case-definitions of the Fetal Alcohol Spectrum Disorder (FASD) 4-digit code. Note: The 3 facial features of Fetal Alcohol Spectrum (FAS) are palpebral fissure lengths 2 or more Standard Deviation (SDs) below the mean and a rank 4 or 5 smooth philtrum and thin upper lip using lip-philtrum guides 1 or 2; Central Nervous System (CNS) rank 1: Unlikely CNS damage is selected when there is no evidence of dysfunction; CNS rank 2: Possible damage is selected when there is moderate dysfunction; CNS rank 3: Probable damage is selected when there is severe dysfunction defined as 3 or more domains of brain function 2 or more SDs below the mean; CNS rank 4: Definite damage is selected when structural/neurological abnormalities are present; Alcohol rank 3: Reflects a confirmed prenatal alcohol exposure at a level less than rank 4 or at a level that is unknown; Alcohol rank 4: Reflects an exposure pattern consistent with the medical literature placing the fetus at high risk.
| Category | Diagnostic name and codes |
|---|---|
| A | Fetal alcohol syndrome (alcohol exposed) |
| 2433 2434 2443 2444 3433 3434 3443 3444 | |
| 4433 4434 4443 4444 | |
| B | Fetal alcohol syndrome (alcohol exposure unknown) |
| 2432 2442 3432 3442 4432 4442 | |
| C | Partial fetal alcohol syndrome (alcohol exposed) |
| 1333 1334 1343 1344 1433 1434 1443 1444 2333 2334 2343 2344 | |
| 2333 2334 2343 2344 3333 3334 3343 3344 4333 4334 4343 4344 | |
| E | Sentinel physical finding(s)/static encephalopathy (alcohol exposed) |
| 3133 3134 3143 3144 3233 3234 3243 3244 | |
| 4133 4134 4143 4144 4233 4234 4243 4244 | |
| F | Static encephalopathy (alcohol exposed) |
| 1133 1134 1143 1144 1233 1234 1243 1244 | |
| 2133 2134 2143 2144 2233 2234 2243 2244 | |
| G | Sentinel physical finding(s)/neurobehavioral disorder (alcohol exposed) |
| 1323 1324 1423 1424 2323 2324 2423 2424 3123 3124 3223 3224 | |
| 3323 3324 3423 3424 4123 4124 4223 4224 4323 4324 4423 4424 | |
| H | Neurobehavioral disorder (alcohol exposed) |
| 1123 1124 1223 1224 2123 2124 2223 2224 | |
| I | Sentinel physical finding(s) (alcohol exposed) |
| 1313 1314 1413 1414 2313 2314 2413 2414 3113 3114 3213 3214 | |
| 3313 3314 3413 3414 4113 4114 4213 4214 4313 4314 4413 4414 | |
| J | No physical findings or CNS abnormalities detected (alcohol exposed) |
| 1213 1214 2113 2114 2213 2214 |
Note: CNS: Central Nervous System.
Table 1: 4-digit diagnostic codes within each diagnostic category.
FASD 4-digit code
All subjects were diagnosed by interdisciplinary teams in WA and AK using a comprehensive, validated diagnostic system called the FASD 4-digit code, depicted in Figure 1 [7,8]. Briefly, the 4 digits of the FASD 4-digit code reflect the magnitude of expression of the 4 key diagnostic features of FASD, in the following order: 1. growth deficiency, 2. FAS facial phenotype, 3. CNS structural/functional abnormalities and 4. Prenatal Alcohol Exposure (PAE). The magnitude of expression of each feature is ranked independently on a 4-point Likert scale, with 1 reflecting complete absence of the FASD feature and 4 reflecting a strong “classic” presence of the FASD feature. Each Likert rank is specifically case defined [7]. 4-digit codes range from 1111 to 4444. Each 4-digit diagnostic code falls into 1 of 22 unique clinical diagnostic categories (labeled A through V). Seven of the 22 diagnostic categories (4-digit categories A-C and E-H) fall broadly under the umbrella of FASD (A: FAS/ Alcohol exposed; B: FAS/alcohol exposure unknown; C: Partial FAS/alcohol exposed; E&F: Static encephalopathy/ alcohol exposed and G&H: Neurobehavioral disorder/alcohol exposed). Diagnostic categories I and J are also included in this study for they reflect the subset of patients with confirmed PAE that present only with growth deficiency and/or the FAS facial features (I: Sentinel physical findings/alcohol-exposed) or present with normal outcomes (J: No sentinel physical findings or CNS abnormalities/alcohol-exposed). Categories I and J are not considered under the umbrella of FASD in accordance with the FASD 4-digit code.
Prenatal alcohol exposure
Measures of prenatal alcohol exposure were obtained from three sources. The first source was each patient’s 4-digit code prenatal alcohol exposure 4-point rank documented at the time of their FASD diagnostic evaluation (Figure 1) [7,8]. Alcohol exposure histories reported to the clinics typically come from the birth mother, other family members and/or past medical and social service records. The second and third sources of PAE were obtained from two national CDC surveillance systems, the Pregnancy Risk Assessment Monitoring System (PRAMS) and Behavioral Risk Factors Surveillance System (BRFSS) [17- 21]. The PRAMS and BRFSS surveillance systems document the annual prevalence of alcohol use during pregnancy across representative samples of each State’s general population. Over the decades, some of the questions on the PRAMS and BRFSS surveys changed preventing one from tracking the same alcohol metric across the entire time period 1991-2015. In addition, not all States chose to include the same alcohol metrics in their annual surveys. PRAMS and BRFSS PAE metrics that were available from both States over the same period of years are presented in this study.
Analyses
Descriptive analyses (proportions) were used to graphically summarize the sociodemographics, FASD diagnostic outcomes and reported prenatal alcohol exposures for each State. It was not the intent of this study to hypothesize and empirically assess how exposures and FASD outcomes may differ between the two states or change over time. The FASD diagnostic outcomes are derived from clinical populations that are typically not representative of the general population. For example, hypothetically, if the prevalence of FAS was higher in the WA clinical population than the AK clinical population, that would not necessarily mean WA had a higher prevalence of FAS in its general population. There are many factors that influence which individuals request and receive an FASD diagnosis (access to diagnostic services, knowledge of prenatal alcohol exposure, caregiver willingness to participate in a FASD diagnostic evaluation, medical insurance coverage, etc.). One factor that is not influencing potential contrasts between the WA and AK FASD outcomes is their use of a single FASD diagnostic system, the FASD 4-digit code. In contrast to the FASD diagnostic outcomes, the prenatal alcohol exposure data generated by the CDC PRAMS and BRFSS surveillance programs are designed to be representative of each state’s general population. The purpose of including these measures of PAE was to provide a context or background from which to qualitatively interpret the WA and AK FASD outcomes, visually compare the drinking patterns between the two States and allow Readers to compare the drinking patterns documented in WA and AK to other States participating in PRAMS and/or BRFSS. Empirically contrasting the WA and AK PRAMS and BRFSS prenatal alcohol exposure patterns would warrant a separate study.
Objective 1: Establishment and description of the WA and AK Statewide FASD diagnostic clinical networks
Washington state: The term FAS was first coined in WA State at the University of Washington in 1973 [22,23]. Over the next 50 years, WA State engaged in an extensive, multifaceted effort to screen, diagnose, treat, track and ultimately prevent FASD. A comprehensive history of these efforts is posted on the WA State FASD website [24]. Although diagnostic services in WA commenced as early as 1973, diagnosis was typically conducted by a geneticist or dysmorphologist relying largely on clinical impression of physical features. In 1993, the University of Washington opened the first interdisciplinary FASD diagnostic clinic sponsored by the CDC as a FASD primary prevention study [2,3,5]. In 1995, the WA Legislature through Senate Bill 5688 expanded the clinic into a statewide network of FASD diagnostic clinics (Figure 2) led by the core clinical/research/ training clinic at the University of Washington in Seattle, WA [11]. Susan J. (Astley) Hemingway, Ph.D., Professor of Epidemiology/Pediatrics, directs the FASDPN. All WA FASDPN clinics use an interdisciplinary approach to diagnosis guided by the FASD 4-digit diagnostic code and submit data to the core clinic at the University of Washington with patient consent and human subject’s approval. The interdisciplinary team includes a medical doctor, psychologist, speech language pathologist, occupational therapist, social worker, family advocate and public health professional. All team members complete the FASD 4-digit code online course [15]. Individuals across the lifespan with a confirmed prenatal alcohol exposure at any level or the full rank 4 FAS facial phenotype are eligible for an evaluation. A FASD diagnostic evaluation is conducted in a single 4-hour evaluation. In preparation for the evaluation, all birth, medical, school and social service records are collected on the patient. These records provide important data (growth, medical, neuropsychological test scores, other adverse prenatal/ postnatal exposures) that are critical to deriving an accurate FASD diagnosis. On the day of the evaluation, the caregivers participate in a joint interview with the medical doctor and social worker while the patient is assessed by the psychologist, speech- language pathologist and occupational therapist. Standardized neuropsychological assessments are administered to assess all aspects of brain function (cognition, executive function, memory, language, attention, adaptation, motor, sensory, etc.). Height, weight, head circumference and facial features are also measured. The team uses data collected in the clinic as well as the data from past records to derive the FASD 4-digit code. They also compose a comprehensive intervention plan addressing medical, behavioral, educational, placement and safety issues. The team shares the diagnosis and intervention plan with the family and submits an 8 to 10-page comprehensive medical summary to the patient’s medical record. All phases of data collection and report generation are documented on standardized, electronic templates available on the FASDPN website [25]. The team conducts two diagnostic evaluations per clinic, one in the morning and one in the afternoon. Twenty years of patient surveys confirmed the FASDPN interdisciplinary diagnosis model using the 4-digit code affords patients and their families substantial access to interventions that meet their needs across the spectrum of FASD diagnoses [26]. The mission of the FASDPN is prevention of FASD through diagnosis, intervention, screening, surveillance, training, public health policy and research. The WA State FASDPN, now in its 30th year, has achieved each of these missions [27]. The FASDPN has diagnosed over 3,000 patients with PAE across the lifespan. It has also established one of the world’s largest clinical/research databases with patient consent, leading to over 100 peer- reviewed publications [28,29]. The WA FASDPN has expanded both nationally and internationally through the training of over 1,700 clinicians in 35 countries. The FASDPN has provided FASD training to over 10,000 WA State community professionals over the 30 years with particular focus on student professionals commencing their careers in public service [30].
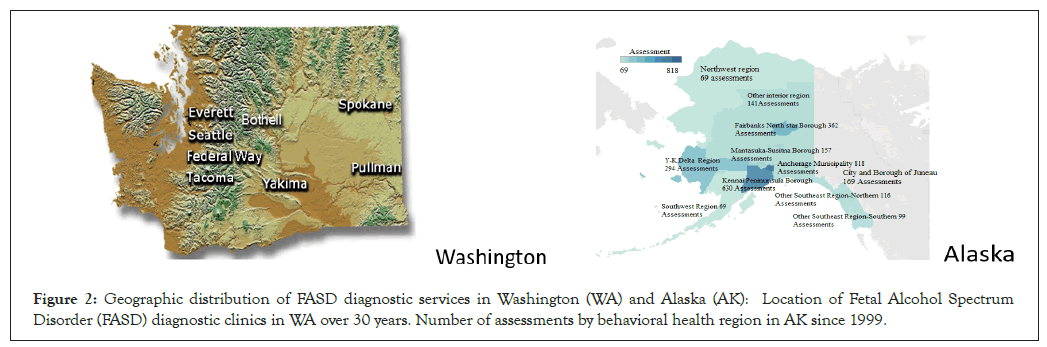
Figure 2: Geographic distribution of FASD diagnostic services in Washington (WA) and Alaska (AK): Location of Fetal Alcohol Spectrum Disorder (FASD) diagnostic clinics in WA over 30 years. Number of assessments by behavioral health region in AK since 1999.
Alaska state: Since 1999, the Alaska Department of Health and Social Services (DHSS), now the Alaska Department of Health, has coordinated and funded a statewide network of community-based FASD diagnostic teams [12]. The number and location of FASD diagnostic teams around the State has varied over time as shown in Figure 2. Originally, 17 teams were trained to serve across the State. In 2023, there are three teams. Diagnosis of FASD is conducted by these interdisciplinary teams using the FASD 4-digit diagnostic code [7]. Team members are trained by completing the FASD 4-digit code online course [15]. Ideally within this model, an interdisciplinary team of clinicians (i.e., medical provider, psychologist, speech language pathologist, occupational therapist and family advocate) is required to diagnose FASD because the damage caused by prenatal alcohol exposure impacts all aspects of an individual’s growth and brain development. Challenges with maintaining all these professionals on a team, especially in rural settings, have required flexibility in functioning [12]. In the event a psychologist, speech language pathologist, or occupational therapist is not available, teams have been allowed to use testing and assessment information from professionals with similar assessment skills and from service entities that may be involved with the individual (e.g., school Individualized Education Program (IEP) documents, other private practice professionals’ reports, etc.).
The expertise of a medical provider is required to asses and interpret the physical and neurological components of the disorder (i.e., growth deficits, facial anomalies, seizure disorders). The expertise of a psychologist, speech language pathologist and occupational therapist is required to assess the CNS functional component of the disorder as described above. Team members are trained by completing the FASD 4-digit code online course [15]. Over the past 20 years, there have been an estimated 3,000 to 4,000 individuals evaluated by the FASD diagnostic teams; assessment data for about 3,000 of these individuals were reported to the State of Alaska [12].
Objective 2: WA and AK FASD outcomes and prenatal alcohol exposures
Sociodemographics: The distribution of patient age at diagnosis, type of caregiver at diagnosis, sex at birth and race are compared between the 2,469 individuals evaluated in Alaska and the 2,532 individuals evaluated in WA as shown in Figure 3. AK and WA evaluated individuals across the lifespan with the majority between 6.0 and 12.9 years of age. WA evaluated a higher proportion (14.9%) of infant/toddlers (birth to 2.9 years of age) than AK (4.2%). Both States evaluated a comparably higher proportion of males than females. The racial distribution of the patient populations cannot be meaningfully compared between the two States because 53.7% of the patients from Alaska did not have race recorded. Whites appeared to be underrepresented (47.8%) in the clinical population evaluated in the WA FASDPN clinics when compared to the 2020 census for WA (7.7 million total population: White (alone) 77%, American Indian/Alaska Native (alone) 2%, Black (alone) 5%, other (alone) and mixed race 16%). For reference, the 2020 census for AK was as follows: (0.7 million total population: White (alone) 65%, American Indian/Alaska Native (alone) 16%, Black (alone) 4%, other (alone) and mixed race 15%). The majority of patients evaluated in both States were in out-of-home care at the time of diagnosis with only 10.3% and 17.2% still in the care of their birth mother in AK and WA respectively [31].
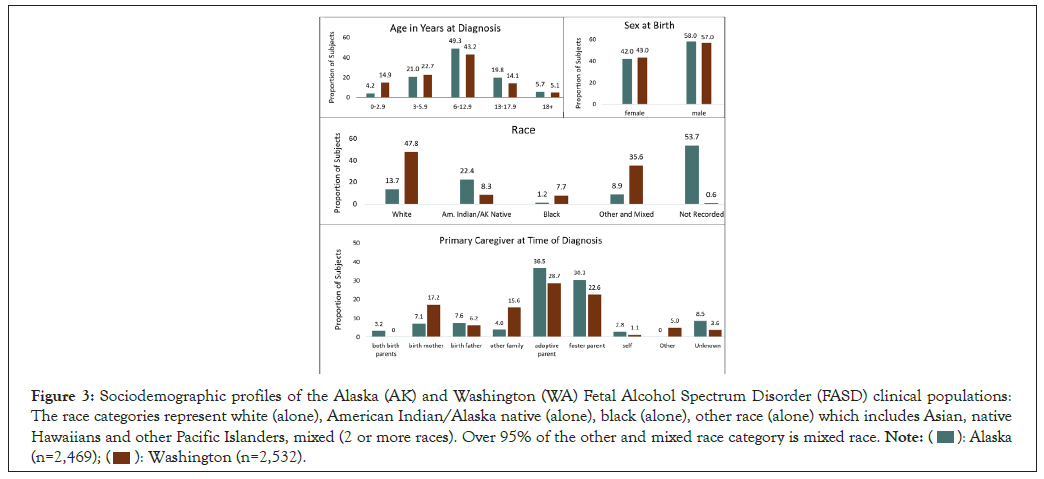
Figure 3: Sociodemographic profiles of the Alaska (AK) and Washington (WA) Fetal Alcohol Spectrum Disorder (FASD) clinical populations:
The race categories represent white (alone), American Indian/Alaska native (alone), black (alone), other race (alone) which includes Asian, native
Hawaiians and other Pacific Islanders, mixed (2 or more races). Over 95% of the other and mixed race category is mixed race. 
Growth, face, CNS and alcohol ranks: The distribution of the 4-digit code ranks for growth, face, CNS and prenatal alcohol exposure for the AK and WA clinical populations are presented in Figure 4. The proportion of patients presenting with moderate and high PAE (ranks 3,4) was near identical between the two States. Roughly 20-30 percent of the AK and WA patients presented with mild to severe growth deficiency (height and/or weight at or below the 10th percentile). The moderate to severe expression of the FAS facial phenotype (ranks 3, 4) was observed in 14 to 30 percent of the two clinical populations. The WA clinical population was more likely to present with moderate CNS dysfunction (52.2% CNS rank 2) than severe CNS dysfunction (22.7% CNS rank 3). In contrast, the AK clinical population was more likely to present with severe CNS dysfunction (47.5% CNS rank 3) than moderate CNS dysfunction (32.5% CNS rank 2). This is explained in part by the higher proportion of infant/toddlers (birth to 3 years of age) attending the WA clinics (14.9%) relative to the AK clinics (4.2%). Infants/toddlers are less likely to meet criteria for severe CNS dysfunction because they are too young to assess higher order brain functions like memory, executive function and language that have not yet fully matured. Severe CNS dysfunction (rank 3) requires 3 or more domains of dysfunction 2 or more SDs below the mean. Moderate CNS dysfunction (rank 2) requires only 1 or 2 domains of dysfunction 2 or more SDs below the mean or multiple domains 1.5 SDs below the mean.
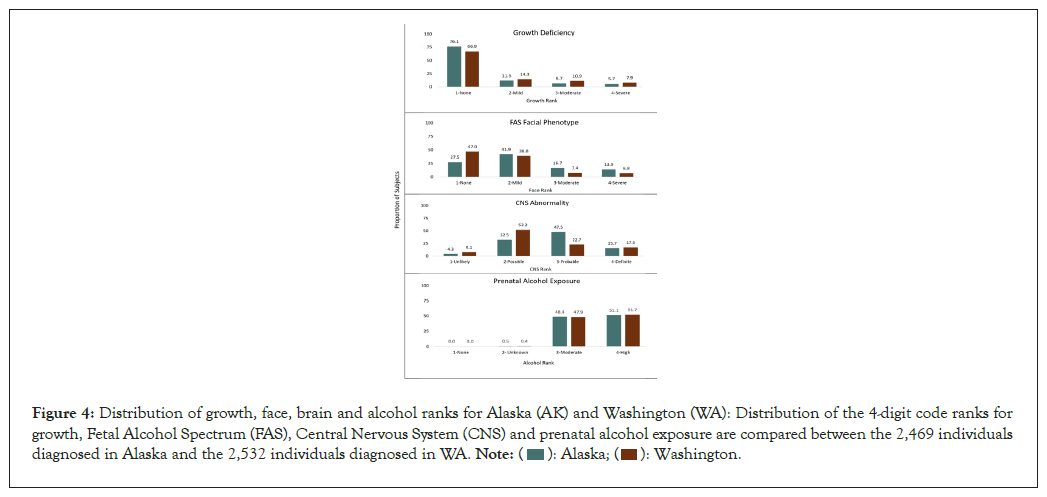
Figure 4: Distribution of growth, face, brain and alcohol ranks for Alaska (AK) and Washington (WA): Distribution of the 4-digit code ranks for
growth, Fetal Alcohol Spectrum (FAS), Central Nervous System (CNS) and prenatal alcohol exposure are compared between the 2,469 individuals
diagnosed in Alaska and the 2,532 individuals diagnosed in WA. 
Prenatal alcohol exposure: The annual prevalence of alcohol use during pregnancy appeared fairly comparable between AK and WA over the decades based on data from two Statewide CDC surveillance systems Pregnancy Risk Assessment Monitoring System and Behavioral Risk Factors Surveillance Systems and the 4-Digit Code prenatal alcohol exposure Rank based on records available at the time of the FASD diagnostic evaluations (Figure 4 and 5) [7,8,17-21]. The PRAMS and BRFSS Statewide surveillance systems strive to document alcohol use during pregnancy across representative samples of the general population. The prevalence of any level of alcohol use during pregnancy reported annually to PRAMS by both States decreased from 1993-1998 (Figure 5A). This decrease is discussed in more detail below under objective 3B. The decrease in both States coincided with the establishment of the WA and AK FASD diagnostic and prevention programs that included substantial statewide efforts to educate the public about the risks of drinking during pregnancy [12,27]. By 1994, both States met or exceeded the healthy people 2000 goal of no more than 10% of women drinking during pregnancy. AK went on to meet the health people 2010 goal of no more than 6% of women drinking during pregnancy from 2000-2010 [32]. WA experienced an increase in drinking from 2000-2010 but remained below the healthy people 2000 goal of 10%. Neither State met the healthy people 2020 goal of 1.7% by 2015. In 2016, the CDC PRAMS survey was revised to document heavy drinking (>8 drinks per week) three months prior to pregnancy, a metric that likely reflects prenatal alcohol exposure before a woman knows she is pregnant (Figure 5B). The prevalence of heavy drinking (>8 drinks per week) three months prior to pregnancy between 2016 and 2020 was comparably stable (2% to 4%) between the two States as documented by PRAMS [17]. The proportion of pregnant women reporting any level of drinking between 2005 and 2021 was higher in WA than AK and closely reflected outcomes documented by PRAMS in the same time period (Figure 5C). The proportion of pregnant women reporting binge drinking (>4 drinks per occasion) appeared to increase from 1% to 5% between 2006 and 2021 in both States (Figure 5D) [20]. Once again, the data collected by PRAMS and BRFSS appeared roughly comparable across the years. The CDC healthy people 2020 target for pregnant women abstaining from binge drinking in the past 30 days was 100%. Comparable levels of prenatal alcohol exposure were also observed between the WA and AK FASD diagnostic clinic populations. Fifty-one percent of all patients evaluated in each State’s FASD diagnostic clinics had documented high prenatal alcohol exposure (4-digit code rank 4) (Figure 4). When these patients were sorted by the year they were born (1991 through 2015), the proportion with high rank 4 exposure within each birth cohort was comparable between both States, fluctuating between 45% to 55%. (Figure 5E) [33,34].
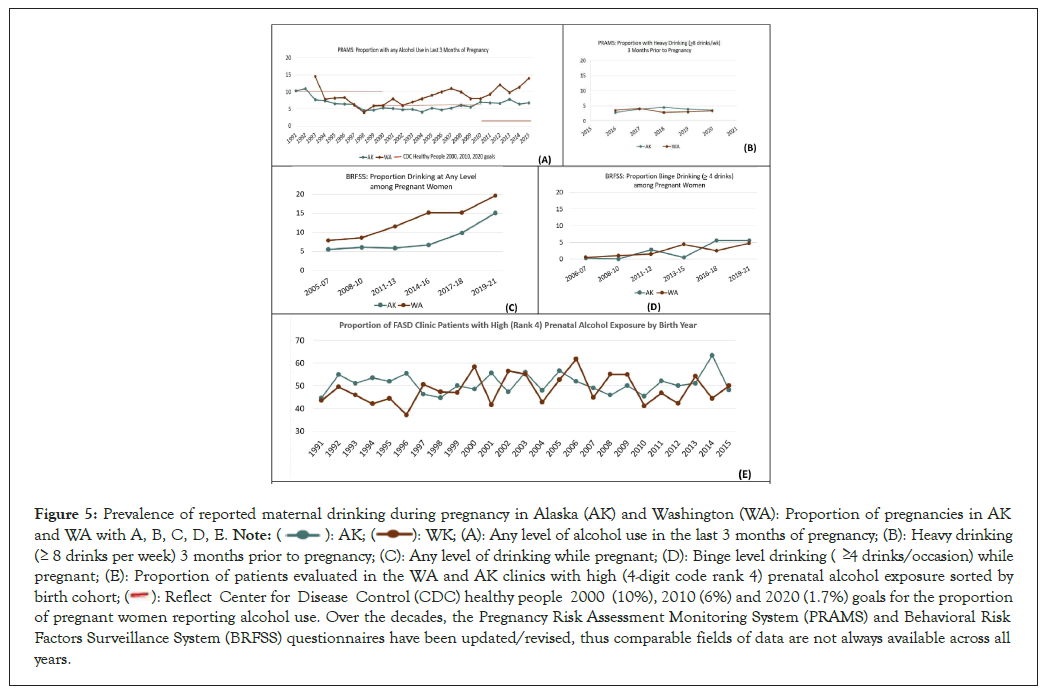
Figure 5: Prevalence of reported maternal drinking during pregnancy in Alaska (AK) and Washington (WA): Proportion of pregnancies in AK and WA with A, B, C, D, E.  (A): Any level of alcohol use in the last 3 months of pregnancy; (B): Heavy drinking
( 8 drinks per week) 3 months prior to pregnancy; (C): Any level of drinking while pregnant; (D): Binge level drinking ( 4 drinks/occasion) while pregnant; (E): Proportion of patients evaluated in the WA and AK clinics with high (4-digit code rank 4) prenatal alcohol exposure sorted by
birth cohort;
(A): Any level of alcohol use in the last 3 months of pregnancy; (B): Heavy drinking
( 8 drinks per week) 3 months prior to pregnancy; (C): Any level of drinking while pregnant; (D): Binge level drinking ( 4 drinks/occasion) while pregnant; (E): Proportion of patients evaluated in the WA and AK clinics with high (4-digit code rank 4) prenatal alcohol exposure sorted by
birth cohort;  2010 (6%) and 2020 (1.7%) goals for the proportion
of pregnant women reporting alcohol use. Over the decades, the Pregnancy Risk Assessment Monitoring System (PRAMS) and Behavioral Risk
Factors Surveillance System (BRFSS) questionnaires have been updated/revised, thus comparable fields of data are not always available across all
years.
2010 (6%) and 2020 (1.7%) goals for the proportion
of pregnant women reporting alcohol use. Over the decades, the Pregnancy Risk Assessment Monitoring System (PRAMS) and Behavioral Risk
Factors Surveillance System (BRFSS) questionnaires have been updated/revised, thus comparable fields of data are not always available across all
years.
FASD diagnostic outcomes by age: FASD diagnostic outcomes by age group for WA and AK are presented in Figure 6. The proportion of patients with FAS appears comparable between WA and AK and comparable between each age group ranging from 2.0% to 8.3%. AK documented a higher proportion of PFAS (15% to 24%) across all age groups relative to WA (4% to 6%). Both States documented a higher proportion of ND/ AE than SE/AE among young patients and a higher proportion of SE/AE than ND/AE among older patients. This would be expected since patients under 8-9 years of age are too young to assess higher order brain functions like memory, executive function and language that have not yet fully matured. A diagnosis of Static Encephalopathy/Alcohol-Exposed (SE/AE) requires standardized psychometric evidence of three or more domains of brain function, 2 or more Standard Deviations (SDs) below the mean. In contrast, a diagnosis of Neurobehavioral Disorder/Alcohol-Exposed (ND/AE) requires 1 to 2 domains of brain function 2 SDs below the mean or multiple domains 1.5 SDs below the mean.
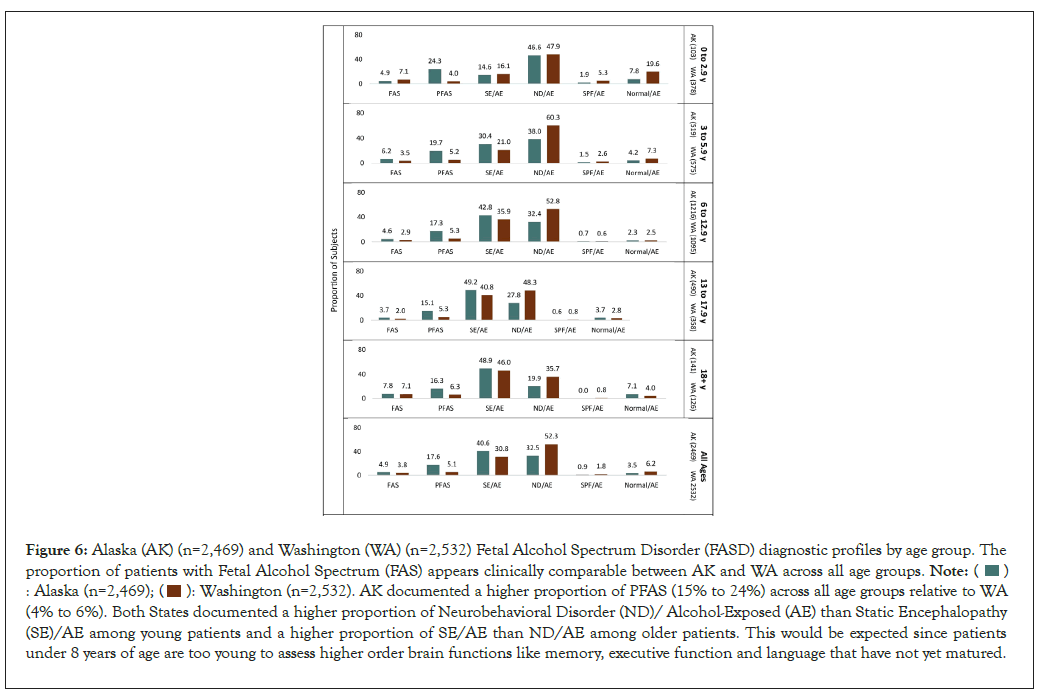
Figure 6: Alaska (AK) (n=2,469) and Washington (WA) (n=2,532) Fetal Alcohol Spectrum Disorder (FASD) diagnostic profiles by age group. The
proportion of patients with Fetal Alcohol Spectrum (FAS) appears clinically comparable between AK and WA across all age groups. 
 AK documented a higher proportion of PFAS (15% to 24%) across all age groups relative to WA
(4% to 6%). Both States documented a higher proportion of Neurobehavioral Disorder (ND)/ Alcohol-Exposed (AE) than Static Encephalopathy
(SE)/AE among young patients and a higher proportion of SE/AE than ND/AE among older patients. This would be expected since patients
under 8 years of age are too young to assess higher order brain functions like memory, executive function and language that have not yet matured.
AK documented a higher proportion of PFAS (15% to 24%) across all age groups relative to WA
(4% to 6%). Both States documented a higher proportion of Neurobehavioral Disorder (ND)/ Alcohol-Exposed (AE) than Static Encephalopathy
(SE)/AE among young patients and a higher proportion of SE/AE than ND/AE among older patients. This would be expected since patients
under 8 years of age are too young to assess higher order brain functions like memory, executive function and language that have not yet matured.
FASD diagnostic outcomes by race: FASD diagnostic outcomes by race for WA and AK are presented in Figure 7. The prevalence of FASD diagnostic outcomes appeared largely comparable across the different races within each State.
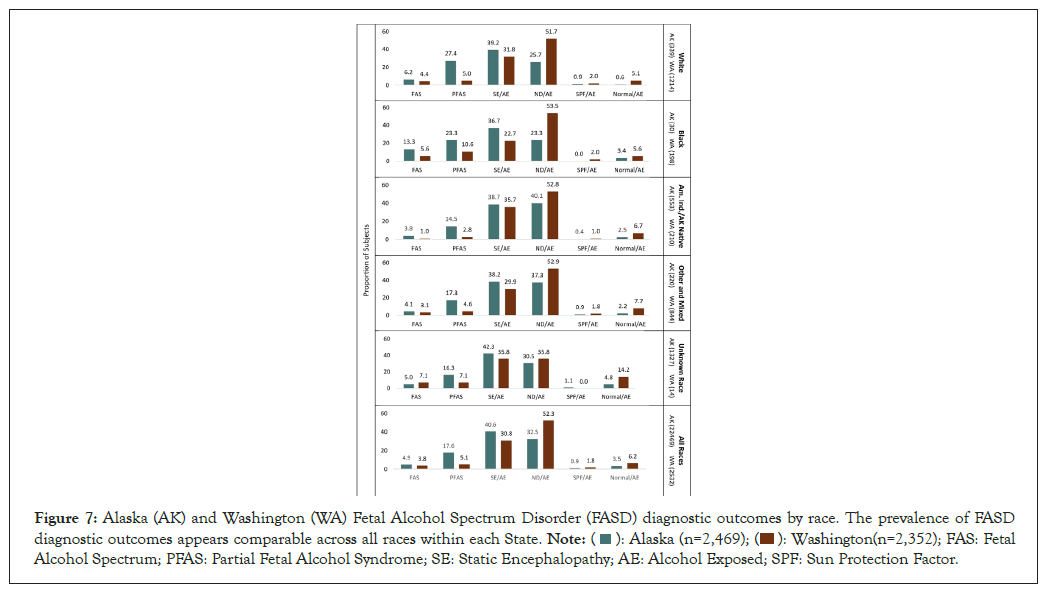
Figure 7: Alaska (AK) and Washington (WA) Fetal Alcohol Spectrum Disorder (FASD) diagnostic outcomes by race. The prevalence of FASD diagnostic outcomes appears comparable across all races within each State.  FAS: Fetal
Alcohol Spectrum; PFAS: Partial Fetal Alcohol Syndrome; SE: Static Encephalopathy; AE: Alcohol Exposed; SPF: Sun Protection Factor.
FAS: Fetal
Alcohol Spectrum; PFAS: Partial Fetal Alcohol Syndrome; SE: Static Encephalopathy; AE: Alcohol Exposed; SPF: Sun Protection Factor.
Distribution of FASD diagnoses by year: The distribution of FASD diagnoses by year for WA and AK are presented in Figure 8. The distribution of FASD diagnoses remained relatively similar from year to year within each State despite the fact that the number of FASD clinics varied annually as did the clinicians providing the diagnostic services. AK observed a higher prevalence of PFAS and lower prevalence of ND/AE than WA. The cumulative proportion of patients with the most severe outcomes (FAS, PFAS and SE/AE) hovered around 60% annually in AK compared to 40% annually in WA.
Objective 3A: Guiding public health policy and legislation to support individuals with FASD
Data collected by the WA FASDPN was instrumental in helping guide public health policies to better meet the needs of individuals with FASD and their families. AK also implemented numerous public health policies over the decades that together, with the establishment of their FASD diagnostic clinics, helped meet the needs of individuals impacted by FASD. Below are a few examples of these policies and legislation. More comprehensive summaries are available from, the Alaska Mental Health Trust Authority and the Alaskan UAA College of Health [12,13,27].
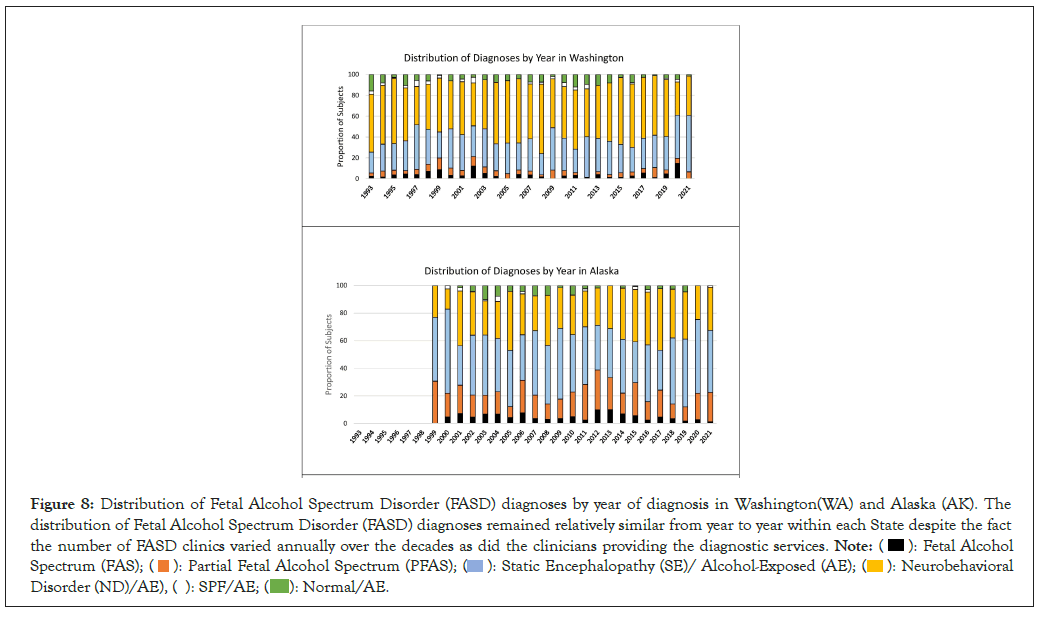
Figure 8: Distribution of Fetal Alcohol Spectrum Disorder (FASD) diagnoses by year of diagnosis in Washington(WA) and Alaska (AK). The
distribution of Fetal Alcohol Spectrum Disorder (FASD) diagnoses remained relatively similar from year to year within each State despite the fact
the number of FASD clinics varied annually over the decades as did the clinicians providing the diagnostic services.

Washington state:
• 1997: Expansion of the CDC-sponsored University of Washington FASD interdisciplinary diagnostic clinic into the Statewide network of clinics (FASDPN) through Substitute Senate Bill 5688 [11]. The WA FASDPN has been funded by the WA state legislature for 30 consecutive years. The Senate Bill also established the WA FASD interagency workgroup ensuring coordination of FASD programs across the Department of Social & Health Services, Superintendent of Public Instruction, Department of Corrections and Department of Health. senate Bill 5688 required the core clinic at the University of Washington to collect data from all FASDPN network clinics and share an annual report with the above-named State departments summarizing the demand for diagnostic services, the ability to meet the demand and the PAE and FASD profiles of the patients served. These outcomes are also shared in aggregate, open-access form via our Tableau interactive dashboards [28].
• 1997-2009: Establishment of a Statewide evidence-based FASD prevention program (parent-child assistance program), evidence-based FASD intervention program (families moving forward) and caregiver advocacy program (FASD focus NW) [35-37].
• 2001: WA State law requiring warning signs from the Liquor Control Board at all points-of-purchase for alcohol warning against the possible danger of birth defects which may be caused by consumption of alcohol during pregnancy Washington Administrative Code 314-11-060 [38].
• 2014: Comprehensive report to the WA state legislature on achievements, current challenges and recommended solutions to screen, diagnose, treat and prevent FASD [27].
• 2015: Acceptance of FASD as a qualifying condition for developmental disability assistance [39].
• 2022: Elimination of the use of IQ scores when determining eligibility for developmental disability assistance. WA second substitute House Bill 2008 [40].
• 2023: An act relating to expanding prevention services, diagnoses, treatment and support for prenatal substance exposure including prenatal alcohol exposure. WA second substitute House Bill 1168 [41].
Alaska state:
• 1999: Alaska Senator Ted Stevens, Chairman of the Senate Appropriations Committee added $29 million into the federal budget to target FASD in Alaska for five years. A massive diagnostic effort began in 2000. The State created FASD diagnostic teams- from Sitka to Barrow, Fairbanks to Bethel to diagnose children with FASD. Seventeen were funded [14].
• 2001: Alaska Statute requiring bars, liquor stores and restaurants to display 11-by-14-inch signs that drinking while pregnant can lead to birth defects [42].
• 2012: The Alaska State Legislature though Senate Bill 151 made an FASD diagnosis a mitigating circumstance to be considered in sentencing for felony level criminal offenses through [43].
• 2012: The Alaska state legislature permanently recognized September 9 as FASD Awareness Day in Alaska through Senate Bill 127. September 9 is recognized worldwide as FASD awareness day [44].
• 2018: Alaska FASD strategic plan 2017-2022. Governor’s Council on Disabilities and Special Education (January 29, 2018). Included in the report is a detailed history of FASD efforts in Alaska from the 1970s through 2022 [14].
• 2020: Alaska FASD diagnostic team data analysis, policy and prevention recommendations prepared for the Alaska Mental Health Trust Authority [12].
Objective 3B: Tracking successful prevention efforts
Washington state: To assess the effectiveness of fetal alcohol syndrome prevention efforts, one must be able to estimate accurately the prevalence of fetal alcohol syndrome over time in population-based samples. With the establishment of the WA FASDPN clinics, the development of the FAS Facial Photographic Analysis Software, the creation of the FASD 4-digit diagnostic code, the establishment of the Foster Care Fetal Alcohol Syndrome Screening Program and the collection of PRAMS data on maternal use of alcohol during pregnancy, the tools, methods and infrastructure for assessing the effectiveness of fetal alcohol syndrome primary prevention efforts in Washington State were in place [7,17-19,45,46]. A cross- sectional study was conducted in 2004 to determine whether the prevalence of fetal alcohol syndrome among children in a foster care population, born between 1993 and 1998, decreased with the documented decrease in prevalence of maternal use of alcohol during pregnancy from 1993 and 1998 in Washington State documented through PRAMS [47]. The prevalence of maternal drinking during pregnancy in Washington State declined significantly (p<0.001) from 1993 to 1998 as did the prevalence of fetal alcohol syndrome among foster children born 1993-98 (p<0.03). These observations support the likelihood that FAS prevention efforts in Washington State were working successfully. Fundamental to WA State’s success in creating and implementing FASD screening, diagnostic, intervention and prevention tools and programs over the decades is the ongoing support received from the state legislature. In April 2023, the Governor of WA signed House Bill 1168, an act relating to providing prevention services, diagnoses, treatment and support for prenatal substance exposure [41]. This bill compliments and expands Senate Bill 5688, enacted in 1995 supporting efforts directed at the early identification of and intervention into the problems associated with fetal alcohol exposure through the creation of the Fetal Alcohol Syndrome Diagnostic and Prevention Network [11].
Alaska state: In a 2009 CDC-sponsored study, medical record abstractions were completed for all potential FAS cases reported to the Alaska Birth Defects Registry for children who were at least 6 years of age (birth years 1996-2002) [48]. Data from these abstractions was linked to birth certificates to determine FAS prevalence estimates. From 1996 to 2002, Alaska experienced a 32% decrease in FAS birth prevalence from 19.9 to 13.5 per 10,000 live births (p=0.05). Decline in the overall FAS prevalence was limited entirely to Alaska Native children who experienced a 49% decline from 63.1 to 32.4 per 10,000 live births (p=0.003). The prevalence among non-Native children increased 64% from 3.7 to 6.1 per 10,000 live births (p=0.18). The observed decline occurred in association with several prevention activities: development and sustainability of a network of community- based FASD diagnostic teams; development of university-level FASD curricula and statewide training programs for educators and providers; a Statewide multi-media public awareness campaign; and increased substance use screening in primary care settings. The temporal association of declining FAS prevalence with prevention activities suggests that the interventions played a role.
WA and AK have demonstrated the feasibility of implementing the 4-digit code and the value of establishing longstanding, statewide, interdisciplinary FASD diagnostic clinical networks. FASD diagnostic clinics and the databases derived from them serve as the foundation for FASD screening, diagnosis, intervention, training, research, public health policy and prevention [2,3,7-9,12,13,15,16,26-28,30,36,38,39,41,46-54]. Ongoing legislative support, centralized data collection and use of an evidence-based FASD diagnostic system with online training continue to be key to the ongoing success of these two networks. Interdisciplinary FASD diagnostic clinics using the 4-digit code have been established worldwide [7,15]. Their FASD diagnostic profiles are compared and contrasted on our Tableau interactive dashboards [28]. Although AK and WA have maintained statewide FASD diagnostic clinics for 20-30 years respectively, establishing and maintaining interdisciplinary FASD diagnostic teams are not without challenges. Briefly, challenges include geographic reach, training and turnover of professionals on FASD diagnostic teams, stigma related to those who consume alcohol during pregnancy and their offspring, funding and community education/readiness. Utilizing telemedicine platforms like zoom proved indispensable during the COVID pandemic for remotely connecting clinicians and patients with the core interdisciplinary teams when in- person attendance was not possible. These platforms continue to be useful to address some of the challenges faced in rural communities. Having access to self-paced online diagnostic training programs like the FASD 4-digit code online course greatly facilitated training of new clinical team members [15].
To enhance interest and education related to FASD the University of Alaska College of Health in Anchorage established a 3-credit elective asynchronous course in 2021 for allied health and related professional students entitled Interdisciplinary Approaches to Fetal Alcohol Spectrum Disorders (FASD): Best Practices in Alaska [49]. For comprehensive reviews of WA and AK FASD diagnostic and prevention efforts, challenges, accomplishments and programmatic recommendations, please see the 2014 comprehensive report to the WA State Legislature on achievements, current challenges and recommended solutions to screen, diagnose, treat and prevent FASD prepared by the WA FASD Interagency Work Group , the 2020 Alaska FASD Diagnostic Team Data Analysis, Policy & Prevention Recommendations and 2021 Alaska FASD Data Systems Development: Gaps, Opportunities & Recommendations prepared for the Alaska Mental Health Trust Authority [12,13,27].
WA and AK have demonstrated the feasibility and value of establishing statewide interdisciplinary FASD diagnostic clinical networks using the FASD 4-digit-code. Legislative support, centralized data collection and use of a single, evidence-based FASD diagnostic system with online training have been key to the long-term, ongoing success of these two diagnostic networks. FASD diagnostic outcomes were similar across the 2,469 Alaskan and 2,532 Washington patients diagnosed over 20 and 30 years respectively. Alcohol use reported by pregnant women in WA and AK, as documented by the CDC PRAMS and BRFSS population-based surveillance systems, followed similar annual trajectories from 1991-2020.
Primary prevention of FASD remains the ultimate goal. To assess the effectiveness of FAS prevention efforts, one must be able to accurately estimate the prevalence of FAS over time in population-based samples. With the establishment of the WA FASDPN clinics, the development of the FAS Facial Photographic Analysis Software, the creation of the FASD 4-digit diagnostic code, the establishment of the Foster Care Fetal Alcohol Syndrome Screening Program and the collection of PRAMS data on maternal use of alcohol during pregnancy, the tools, methods and infrastructure for assessing the effectiveness of FAS primary prevention efforts in Washington State were in place. As early as the 1990s, WA documented significant decreases in the prevalence of FAS and PAE. AK also documented a significant decline in the birth prevalence of FAS in the 1990s using medical record review of all cases reported to the Alaska Birth Defects Registry. The decline in FAS occurred in association with the establishment of their FASD diagnostic clinics, statewide training of educators and clinicians and public awareness campaigns. Establishing and maintaining FASD diagnostic and prevention networks are not without challenges. The benefits to families impacted by FASD, however, far outweigh the challenges.
The creation of the WA and AK Statewide clinical datasets would not have been possible without the extensive clinical efforts and support of the interdisciplinary diagnostic teams and community health/social service agencies in WA and AK over the decades. Special thanks are extended to the patients and their families for their benevolent contributions to these public health efforts to help all families impacted by FASD.
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Google Scholar] [PubMed]
Citation: Hemingway SJA, Baldwin M, Pierce-Bulger M (2023) Washington and Alaska Statewide Fetal Alcohol Spectrum Disorder Diagnostic Clinical Networks: Comparison of Three Decades of 4-Digit Code Diagnostic Outcomes and Prenatal Alcohol Exposure Histories. Adv Pediatr Res. 10:070.
Received: 28-Nov-2023, Manuscript No. LDAPR-23-28470; Editor assigned: 30-Nov-2023, Pre QC No. LDAPR-23-28470; Reviewed: 15-Dec-2023, QC No. LDAPR-23-28470; Revised: 22-Dec-2023, Manuscript No. LDAPR-23-28470; Published: 29-Dec-2023 , DOI: 10.35248/2385-4529.23.10.070
Copyright: © 2023 Hemingway SJA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.