Journal of Cell Science & Therapy
Open Access
ISSN: 2157-7013
ISSN: 2157-7013
Research Article - (2023)Volume 14, Issue 1
Stable heavy isotopes of bio-elements have potential biological activities which is an issue being neglected. The enrichment of heavy isotope in the molecule of a substance may alter the substance’s effects on organism. In our study, the enrichment level of heavy carbon isotope (13C) in testosterone reached to the isotopic ratio (13C/12C) of 9.78%, by which heavy carbon testosterone was produced. The human cancer cells of breast (MCF-7), ovarian (SK-OV-3), and endometrial (Ishikawa) were exposed to testosterone and heavy carbon testosterone (named 13C enriched testosterone) respectively. The growth and morphology of the cells were studied. The dose impact of the compound was investigated as well. To the best of our knowledge, this is the first study demonstrating the influence of heavy carbon isotope on cancer cells. The enrichment of heavy carbon isotope reduced growth of the cells. The cell morphology did not change significantly.
Stable isotope; Carbon-13; Testosterone; Breast Cancer Cells; Ovarian Cancer Cells; Endometrial Cancer Cells
Organisms are composed of bio-elements, such as carbon, hydrogen, oxygen, and nitrogen. Most of these stable isotopes are light mass isotopes. Artificial enrichment of heavy isotope in a molecule is used as the tracer in metabolism research. The basic assumption for the tracing is that the biological behavior of a molecule enriched with heavy isotope is the same as that of its natural counterpart. However, the differences in isotopic ratios may influence the physiological and pathological process of organisms. A number of studies on physiology, pathology, and pharmacology have presented the isotope effect to the scientific community [1-7]. The difference in the biological behaviors between light and heavy isotope should be paid attention in biological and clinical research.
Among the biogenic elements, deuterium (D) is believed to exhibit the most obvious isotope effects [2,8]. It was reported that enriched deuterium negatively affected organisms, including decrease of protein and nucleic acids synthesis and disturbance of cell differentiation [9,10]. However, there have been fewer studies on the effect of other stable isotopes in biology than deuterium [10]. The effect of carbon-13 (13C) has seldom been studied. Carbon is the fundamental element of biological molecules. Carbon atoms form linear or circular molecular frame via combining with carbon itself and other biogenic elements, with numerous functional groups integrated into the molecule by bonding with carbon atom. According to the theory of Isotope Kinetic Effect (IKE) [11], changes in the isotopic composition of a reactant potentially affect the related bio-chemical reaction rate. In view of the crucial role of carbon in molecular structure, carbon isotope effect is worth of being studied intensively.
Testosterone (C19H28O2) is an androgen hormone performing numerous physiological functions at normal circulating concentration levels. The disruption of sex hormone action may lead to the development of cancers (such as breast, ovarian, and endometrial cancer) [12]. A number of evidences suggest that androgen correlates with the carcinogenesis of breast cancer [13,14], ovarian cancer [15-18], and endometrial cancer [19,20]. Several studies indicated that androgen prompted cell proliferation and DNA synthesis of breast cancer cells [21,22]. Some in vitro studies linked androgen-related signaling to ovarian cancer through increased cellular proliferation and reduced apoptotic rates [23,24]. The effect of testosterone on the proliferation of endometrial cancer cells (Ishikawa) has rarely been reported. Understanding that heavy isotope enrichment would slow biochemical reactions and retard the growth of organism [25], our hypothesis was that the heavy carbon testosterone could reduce the promotion effect of testosterone on cell growth.
In the present study, 13C enrichment level of 9.78% was used. Cancer cells of breast (MCF-7), ovarian (SK-OV-3), and endometrial (Ishikawa) were cultured for various periods of time. The influence of heavy carbon testosterone on the three cell lines was investigated. The doses of heavy carbon testosterone were around the physiological concentrations. The objective of our study was to display the biological effect of heavy carbon isotope on cancer cells.
Materials and reagents
Human breast cancer cells (MCF-7), human ovarian cancer cells (SK-OV-3), and human endometrial cancer cells (Ishikawa) were from Tianjin Saierbio Co., Ltd. (China). Testosterone and testosterone-2,3,4-13C3 were from Sigma-Aldrich (China) and the chemical structures were showed in Figure 1. Reagents for cell culture, including PBS, DMEM, EDTA, and FBS, were purchased from Thermo Fisher Scientific (China). MTS cell proliferation colorimetric assay kit was from Amyjet Scientific Co. (China).
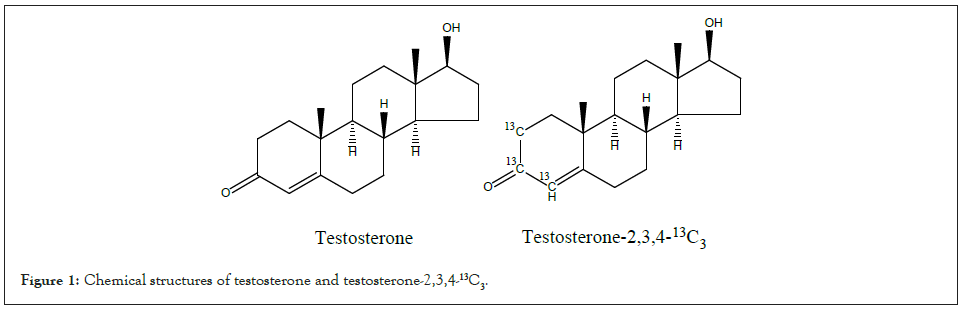
Figure 1: Chemical structures of testosterone and testosterone-2,3,4-13C3.
Production of heavy carbon testosterone
Heavy carbon testosterone with the carbon isotopic ratio of 9.78% was produced by mixing equal amount (in mole) of testosterone (in ethanol solution) and testosterone-2,3,4-13C3 (in ethanol solution) thoroughly. The store solution of 13C enriched testosterone was used to prepare the culture medium containing various concentrations of heavy carbon testosterone.
Cell culture and exposure design
Human cancer cells of breast (MCF-7), ovarian (SK-OV-3), and endometrial (Ishikawa) were cultured in high glucose DMEM medium containing penicillin and streptomycin and 10% FBS. After 3 day culture, 0.25% trypsine-EDTA was utilized to trypsinize the cells which were seeded in the well of 96 well plates (5000 per-well) afterwards. The cells were interfered with heavy carbon testosterone and testosterone, respectively. The doses of exposure were 0, 10-10, 10-9, 10-8, and 10-7 mol/L. These doses included plasma concentrations (10-10 and 10-9 mol/L) [26] and super physiological concentrations (10-8 and 10-7 mol/L) of testosterone for women. Non-drug groups (0 mol/L) were accompanied as blank control.
Cell growth assessment
Cell growth was detected by MTS assay after the cells were exposed to heavy carbon testosterone and testosterone at different time period. One aliquot of phenazine methosulfate solution and 20 aliquot of MTS solution were mixed previously and added to 96 well plates which was incubated for 2 h at 37˚C. The absorbance was measured by a spectrophotometer (BIO-TEK, USA) at the wavelength of 490 nm. The average of Optical Density (OD) values (n=6) was normalized by the control.
Observation of cell morphology
Cell morphology of breast cancer (MCF-7), ovarian cancer (SK- OV-3), and endometrial cancer (Ishikawa) was observed before and after 13C enriched testosterone treatment. The observation was conducted with inverted microscope (Olympus CKX53, Japan).
Statistics
The ANOVA and Post-hoc Test were used to assess significant differences. SPSS 26.0 was the software for the statistical analysis. P<0.05 was considered significant.
Cell growth
The effect of heavy carbon testosterone on cell growth at different doses was analyzed. The OD values by MTS assay were normalized to the control (0 mol/L).
Breast cancer cell
Compared with blank control, the growth activities of breast cancer cells showed no significant differences at 24 h (Figure 2a) but increased with the intervention of testosterone at 48 h (Figure 2b) and 72 h (Figure 2c). These effects were greater at the doses of 10-9 and 10-8 mol/L than at other doses. Compared with blank control, heavy carbon testosterone did not enhance the cell growth at the experiment doses during the experiment period of time (Figure 3). With the intervention of heavy carbon testosterone, no significant differences in these effects among the four doses were observed.
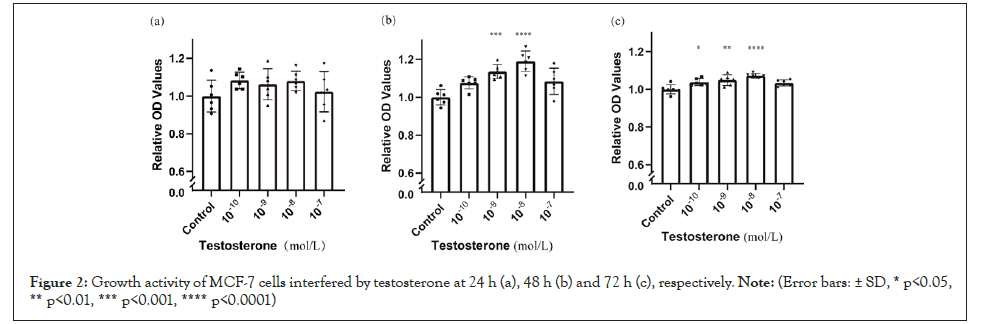
Figure 2: Growth activity of MCF-7 cells interfered by testosterone at 24 h (a), 48 h (b) and 72 h (c), respectively. Note: (Error bars: ± SD, * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001).
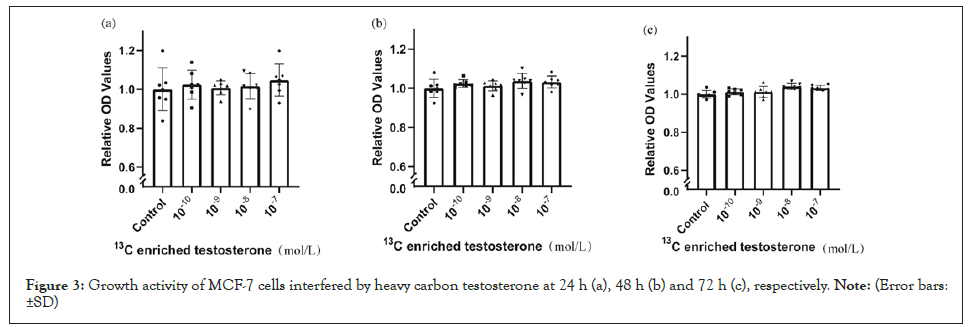
Figure 3: Growth activity of MCF-7 cells interfered by heavy carbon testosterone at 24 h (a), 48 h (b) and 72 h (c), respectively. Note: (Error bars: ±SD).
Ovarian cancer cell
Compared with blank control, the growth of ovarian cancer cells showed no significant differences at 24 h (Figure 4a) but increased with the intervention of testosterone at 48 h (10-8 mol/L) (Figure 4b) and 72 h (10-10-10-7 mol/L) (Figure 4c). Compared with blank control, heavy carbon testosterone did not stimulate the cell growth at various doses during the experiment period of time (Figure 5). There were no significant differences in these effects of heavy carbon testosterone among the four dose groups.
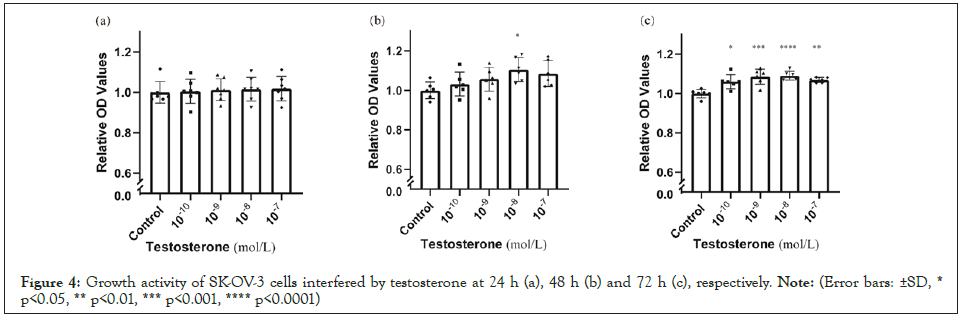
Figure 4: Growth activity of SK-OV-3 cells interfered by testosterone at 24 h (a), 48 h (b) and 72 h (c), respectively. Note: (Error bars: ±SD, * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001).
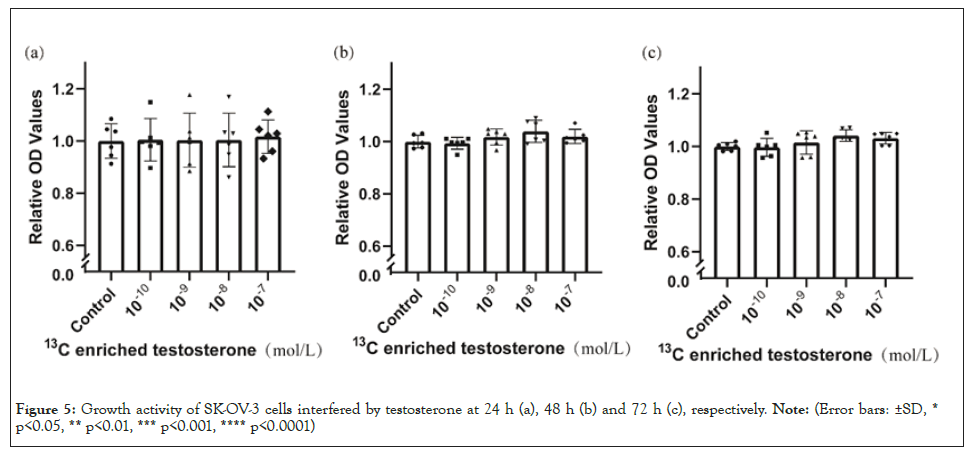
Figure 5: Growth activity of SK-OV-3 cells interfered by testosterone at 24 h (a), 48 h (b) and 72 h (c), respectively. Note: (Error bars: ±SD, * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001).
Endometrial cancer cell
Compared with blank control, there are no significant differences at 24 h (Figure 6a) and 48 h (Figure 6b) but testosterone enhanced the growth of endometrial cancer cells at 72 h (Figure 6c). Compared with blank control, heavy carbon testosterone did not stimulate the cell growth at various doses during the experiment period of time (Figure 7). There were no significant differences in these effects of heavy carbon testosterone among the four dose groups.
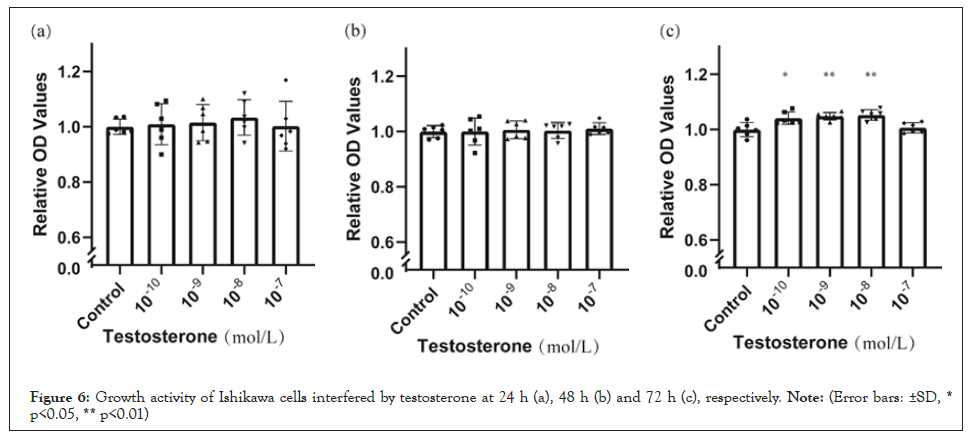
Figure 6: Growth activity of Ishikawa cells interfered by testosterone at 24 h (a), 48 h (b) and 72 h (c), respectively. Note: (Error bars: ±SD, * p<0.05, ** p<0.01).
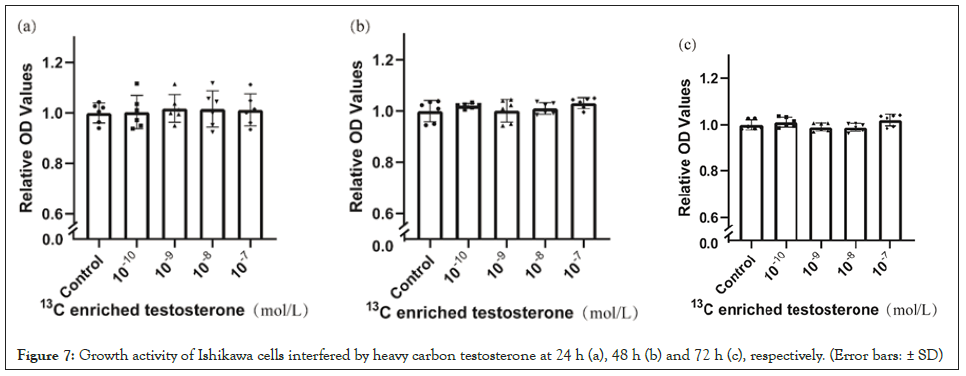
Figure 7: Growth activity of Ishikawa cells interfered by heavy carbon testosterone at 24 h (a), 48 h (b) and 72 h (c), respectively. (Error bars: ± SD).
Cell morphology
Cell morphology of breast cancer (MCF-7), ovarian cancer (SK- OV-3), and endometrial cancer (Ishikawa) cultured with 10-8 mol/L of heavy carbon testosterone at 72 h was observed. The cell morphology of blank group was checked as control (Figure 8). No distinguished differences in cell morphology were observed between the heavy carbon testosterone group and control group.
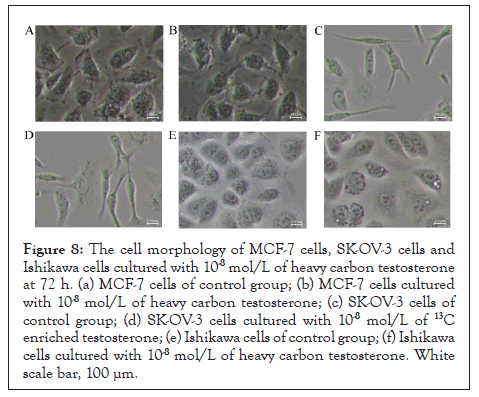
Figure 8: The cell morphology of MCF-7 cells, SK-OV-3 cells and Ishikawa cells cultured with 10-8 mol/L of heavy carbon testosterone at 72 h. (a) MCF-7 cells of control group; (b) MCF-7 cells cultured with 10-8 mol/L of heavy carbon testosterone; (c) SK-OV-3 cells of control group; (d) SK-OV-3 cells cultured with 10-8 mol/L of 13C enriched testosterone; (e) Ishikawa cells of control group; (f) Ishikawa cells cultured with 10-8 mol/L of heavy carbon testosterone. White scale bar, 100 μm.
It has been clarified that large deviation from the intrinsic isotopic composition of organism affects the life process of organism. Besides deuterium, 15N has been investigated for the isotope effect [10,25]. However, the isotope effect of 13C has seldom been reported. Our findings proved that 13C enrichment in a compound would affect the biological behavior of the substance. The present study showed that testosterone stimulated proliferation of the three cancer cell lines at certain doses, whereas heavy carbon testosterone did not enhance the growth. The results consist with KIE theory, i.e., the enrichment of heavy isotope in reactant slows down reaction rate and decreases the development of organism [25]. The present and previous study showed that testosterone prompted proliferation of breast cancer cell MCF-7 in a concentration related manner [13]. In the present study, heavy carbon testosterone acted in a dose independent pattern. This finding is reported for the first time. Cancers of breast, ovarian, and endometrial are related to sex hormone. Some studies indicated that androgen may increase the risk of being attacked by breast cancer [27,28], ovarian cancer [29,30], and endometrial cancer [31]. Androgen, androgen receptor and aromatase enzyme were studied in these studies [30,32- 35]. Androgen receptor is expressed in the cells of breast cancer MCF-7 [32], ovarian cancer SK-OV-3 [36], and endometrial cancer Ishikawa [37], so is aromatase [13,38,39]. It implies that either androgen or estrogen converted from androgen should be responsible for the biology mechanism. In the present study, 13C being labeled at C2, C3, and C4 of testosterone molecule (Figure 1) was assumed to influence the bio-chemical reactions involving in androgen receptors and/or aromatase. The relevant mechanism should be further investigated.
The current study showed that testosterone at physiological level (males 8.8-30.9 nmol/L, females 0.4-2.0 nmol/L) [40] prompted the cancer cell growth, but 13C enriched testosterone at these doses did not. The present results added evidence to the previous findings [41] that stable heavy isotope of carbon has potential biological influence on the organisms.
Heavy carbon testosterone had the effect of reducing the cell growth of breast cancer, ovarian cancer, and endometrial cancer at the experiment conditions. These effects were presented to be dose independent (10-10-10-7 mol/L). Heavy isotope of carbon potentially influenced the biology of organisms.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
[Crossref] [Google Scholar] (All version) [PubMed]
[Crossref] [Google Scholar] (All version) [PubMed]
[Crossref] [Google Scholar] (All version) [PubMed]
[Crossref] [Google Scholar] (All version) [PubMed]
[Crossref] [Google Scholar] (All version) [PubMed]
[Crossref] [Google Scholar] (All version) [PubMed]
[Crossref] [Google Scholar] (All version) [PubMed]
[Google Scholar] (All version) [PubMed]
[Crossref] [Google Scholar] (All version) [PubMed]
[Crossref] [Google Scholar] (All version) [PubMed]
[Crossref] [Google Scholar] (All version) [PubMed]
[Crossref] [Google Scholar] (All version) [PubMed]
[Crossref] [Google Scholar] (All version) [PubMed]
[Crossref] [Google Scholar] (All version) [PubMed]
[Crossref] [Google Scholar] (All version) [PubMed]
[Crossref] [Google Scholar] (All version) [PubMed]
[Google Scholar] (All version) [PubMed]
[Crossref] [Google Scholar] (All version) [PubMed]
[Crossref] [Google Scholar] (All version) [PubMed]
[Crossref] [Google Scholar] (All version) [PubMed]
[Crossref] [Google Scholar] (All version) [PubMed]
[Crossref] [Google Scholar] (All version) [PubMed]
[Crossref] [Google Scholar] (All version) [PubMed]
[Crossref] [Google Scholar] (All version) [PubMed]
[Crossref] [Google Scholar] (All version) [PubMed]
[Crossref] [Google Scholar] (All version) [PubMed]
[Crossref] [Google Scholar] (All version) [PubMed]
[Crossref] [Google Scholar] (All version) [PubMed]
[Crossref] [Google Scholar] (All version) [PubMed]
[Crossref] [Google Scholar] (All version) [PubMed]
[Crossref] [Google Scholar] (All version) [PubMed]
[Crossref] [Google Scholar] (All version) [PubMed]
[Crossref] [Google Scholar] (All version) [PubMed]
[Crossref] [Google Scholar] (All version) [PubMed]
[Crossref] [Google Scholar] (All version) [PubMed]
[Crossref] [Google Scholar] (All version) [PubMed]
[Crossref] [Google Scholar] (All version) [PubMed]
[Crossref] [Google Scholar] (All version) [PubMed]
[Crossref] [Google Scholar] (All version) [PubMed]
[Crossref] [Google Scholar] (All version) [PubMed]
[Crossref] [Google Scholar] (All version)
Citation: Zhang M, Wu X, Yang W, Yang R (2023) 13C of Testosterone Affecting Breast, Ovarian, and Endometrial Cancer Cells. J Cell Sci Therapy.14:376.
Received: 08-Nov-2022, Manuscript No. JCEST-22-20000; Editor assigned: 14-Nov-2022, Pre QC No. JCEST-22-20000 (PQ); Reviewed: 22-Nov-2022, QC No. JCEST-22-20000; Revised: 30-Nov-2022, Manuscript No. JCEST-22-20000 (R); Published: 09-Dec-2022 , DOI: 10.35248/2157-7013.22.14.376
Copyright: © 2023 Zhang M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.