Angiology: Open Access
Open Access
ISSN: 2329-9495
ISSN: 2329-9495
Research Article - (2022)Volume 10, Issue 5
Objective: Compare the clinical data and 18F-FDG PET/CT imaging results of patients with Takayasu Arteritis (TAK) with active disease or who are in clinical remission.
Methods: Forty TAK patients (8 with active disease, 32 in remission) were studied. Intravenous 18F-FDG was administered for imaging, and increased 18F-FDG uptake was considered positive.
Results: All patients in both groups tested positive for TAK based on 18F-FDG PET/CT imaging. The active disease group had a higher TAK NIH score, Erythrocyte Sedimentation Rate (ESR), and level of C Reactive Protein (CRP) (all P<0.001), but the two groups had similar sex ratios, age, and disease duration. Quantitative analysis of changes in the mean standard uptake value (SUVmean) and the maximum SUV (SUVmax) of 40 patients when they had active disease and when they were in clinical remission showed there was an insignificant decrease in SUVmax (2.16 ± 0.46 vs. 2.08 ± 0.49, P=0.56) and an insignificant increase of SUVmean (2.34 ± 0.57 vs. 2.42 ± 0.81, P=0.46). However, the arterial SUVmax was higher in patients with active disease than in those with clinical remission (P<0.001). Overall, 27 patients (68%) had a SUVmax of 2.0 or more and 13 (32.5%) had an SUVmax below 2.0. There were no correlations of the imaging results with the type of treatment, time since remission, or laboratory parameters.
Conclusion: The criteria for clinical remission from TAK were not entirely consistent with the actual vascular inflammatory activity based on 18F-FDG PET/CT can play an important role in monitoring the clinical and radiographic remission of patients with TAK.
Takayasu Arteritis; Remission; 18F-FDG PET/CT; Monitoring
Takayasu Arteritis (TAK) is a rare, chronic, idiopathic, autoimmune, and inflammatory disease that is characterized by granulomatous vasculitis of large vessels in the aorta and its immediate branches, and in the pulmonary artery [1,2]. Diagnosis and the timely and accurate assessment of arteritis severity and recurrence are key to the successful treatment of patients with TAK. The aim of treatment is suppression of inflammation and establishment of a state of remission. Traditional Disease Modifying Antirheumatic Drugs (DMARDs) can provide clinical remission in 30% to 50% of TAK patients, depending on the clinical setting [3,4]. However, there is some controversy regarding the criteria to be used to define remission.
Kerr, et al. [5], proposed criteria for disease activity in 1994, and these were subsequently adopted by the American College of Rheumatology (ACR). These criteria consist of four signs and symptoms, and two or more these must be present for a positive diagnosis [5,6]. Because TAK is rare and patients experience multiple nonspecific clinical symptoms, early diagnosis is often difficult. Thus, previous studies have examined the use of different biomarkers to diagnose and evaluate the disease activity. Imaging techniques are required to monitor the disease course and response to treatment. Increased vascular wall thickness, vascular wall edema, and wall lining enhancement are generally considered evidence of disease activity [7,8].
Studies of patients in east Asia showed that aortic regurgitation, pulmonary hypertension, and an elevated serum level of NT-pro B-type Natriuretic Peptide (NT-proBNP) were associated with higher disease activity [1,9]. However, recent studies reported discrepancies between the results from clinical and laboratory parameters with those from various imaging techniques used to assess vascular inflammation [10]. For example, clinicians commonly use Erythrocyte Sedimentation Rate (ESR) and C-reactive Protein (CRP) to evaluate the arteritis severity, but some studies showed normal levels of these markers in certain patients with active disease [11,12]. Thus, normal levels of ESR and CRP do not necessarily indicate the regression of vascular wall inflammation and the cessation of lesions. In addition, although ESR and CRP are elevated in many patients with TAK, they are not specific markers of vascular inflammation [13-15]. Conventional angiography is considered the gold standard for assessment of vasculitis, but it is invasive and associated with iatrogenic morbidities due to radiation exposure and use of contrast media [16].
Researchers first reported the use of 18F-Fluorodeoxyglucose (18F-FDG) positron emission tomography (PET) for assessment of vasculitis in 1999, and this technique subsequently received widespread acceptance for the diagnosis and monitoring of TAK [17]. Previous research reported that 18F-FDG PET/CT had a sensitivity of 92% and a specificity of 100% in the diagnosis of macrovasculitis [18]. Furthermore, 18F-FDG PET/CT can evaluate the efficacy of drug therapy in patients with TAK, in that decreased vascular absorption is associated with clinical remission [19,20]. It should be noted that some patients who receive treatment with immunosuppressive agents experience relief of clinical symptoms and declines of inflammatory markers, but their 18F-FDG PET/CT results still show an abnormal carotid artery, suggesting the lesion remains present but there was simply a lower level of disease activity [21]. The diagnostic value of 18F-FDG PET/CT is attributed to the increased glucose metabolism in inflamed tissues and the increased uptake of radio-labelled glucose [9]. Even in patients receiving steroid therapy, 18F-FDG PET/CT can distinguish patients with active and inactive diseases. Although newer nuclear medicine technologies have proven effectiveness, their use is limited in many areas due to high cost and limited availability compared to 18F-FDG PET/CT.
In the present study we compared TAK patients who had active disease with TAK patients who were in clinical remission to determine whether the results from clinical and laboratory parameters were consistent with the actual vessel inflammation as determined by 18F-FDGPET/CT.
Characteristics of enrolled patients
From September 2011 to September 2021, 40 patients from a large cohort of TAK patients who received care at the rheumatology outpatient clinic were randomly selected. At the examination, 8 patients had clinically active disease and 32 patients were in clinical remission based on the NIH criteria. Patients were included if they fulfilled the following criteria: (i) Diagnosis of TAK based on the NIH criteria [5], (ii) Disease duration of more than 2 years, (iii) No disease flare during the preceding 6 months, (iv) No change in treatment regimen for at least 6 months for patients in clinical remission. The exclusion criteria were pregnancy, liver or renal function impairment, heart failure, respiratory failure, diabetes mellitus, psychological or neurological disorders, or malignancy. TAK clinical remission was defined according to the preliminary NIH criteria: Absence of new or aggravated systemic and/ or local symptoms and/or signs; blood pressure stabilization; normalization of ESR and CRP; and no new onset or progression of existing lesions based on imaging. This study was approved by the Ethics Committee of Xijing Hospital, and all patients participated voluntarily and signed informed consent. (Approval No. KY20163015-1).
Patient and public involvement statement
How can research questions and outcome measures be developed based on patient priorities, experience, and preferences? This study was an observational clinical study without intervention measures, and the real clinical data were recorded and analyzed. How did you involve patients in the design of the study? All patients who participated in clinical observation signed informed consent and completed follow-up study. Were patients involved in the recruitment and implementation of the study? Study patients who participate in clinical observation participate in the study voluntarily and agree to record and analyze clinical data. How are the findings disseminated to study participants? Participants had the right to know and ask about the results. For randomized controlled trials, is the burden of intervention assessed by patients themselves?
18F-FDGPET/CT image acquisition and analysis
All patients received 18F-FDG PET/CT scans using the same equipment (Biograph 40, Siemens) according to standard clinical protocols [22,23]. Imaging was performed 60 min after intravenous injection of approximately 3.7 MBq (0.1 mCi) per kg bodyweight of 18F-FDG. All patients had blood glucose levels below 200 mg/ dL before injection. PET data were reconstructed iteratively, with and without attenuation correction, based on CT data and reoriented in axial, sagittal, and coronal slices. Objective reference values were obtained by measuring the mean standard uptake value (SUVmean) of the mediastinal blood pool and liver. Vascular-wall 18F-FDG uptake was quantified by drawing a volume of interest (irregular and voxelized) in the ascending aorta, arcus aorta, descending aorta, abdominal aorta, right brachiocephalic artery, right subclavian artery, left subclavian artery, right common carotid artery, and left common carotid artery. The 18F-FDG uptake was quantified as SUVmean in the center of the inferior vena cava. The target/background ratio was calculated as (SUVmax in the arterial wall)/(SUVmean in the inferior vena cava), as previously described [24]. The SUVmax cut-off was set at 2.0 (strong accumulation: SUV>2.0) for detecting active inflammation of TAK in untreated cases, as previously described [25].
Demographic and clinical features
We examined 40 patients who had TAK, 8 who had active disease and 32 who were in remission based on the preliminary NIH criteria (Table 1). There were 2 males (25%) in the active group and 12 males (38%) in the remission group, the mean age was 38 years old in the active group and 37 years old in the remission group, and the mean disease duration was 8.35 months in the active group and 9.35 months in the remission group (all P>0.05). The active group had a higher mean NIH score (2.7 vs. 1, P<0.001), mean ESR (37.3 vs. 4.9 mm/h, P<0.001), and mean CRP (13.5 vs. 3.7 mg/L, P<0.001). All 8 patients with active disease were taking a glucocorticoid with 1 or more DMARD’s, and 28 patients in the remission group (88%) were taking a glucocorticoid alone or a glucocorticoid with 1 or more DMARD’s.
| Variable | Active disease (n=8) | Clinical remission (n=32) |
p-value |
|---|---|---|---|
| Males/Total, n/n | 2/8 | 12/32 | NS |
| Mean age (range), years | 38 (18,40) | 37 (21,38) | NS |
| Mean NIH score (range) | 2.7 (2,4) | 1 (1,1) | <0.001 |
| Disease duration ± SD (range), months | 8.35 ± 1.56 (7-12) | 9.35 ± 1.71 (8-11) | NS |
| Mean ESR ± SD, mm/ha | 37.3 ± 28.77 | 4.89 ± 2.67 | <0.001 |
| Mean CRP ± SD, mg/Lb | 13.52 ± 5.62 | 3.72 ± 2.46 | <0.001 |
| Medication, n (%) | |||
| Glu alone | 0 (0%) | 2 (5%) | NS |
| Glu+MTX+LEF | 2 (25%) | 6 (20%) | NS |
| Glu+CYS+LEF | 2 (20%) | 8 (25%) | NS |
| Glu+CYS | 2 (20%) | 5 (20%) | NS |
| Glu+LEF+TAC | 1 (15%) | 6 (15%) | NS |
| Glu+LEF | 1 (10%) | 1 (5%) | NS |
Abbreviations: NIH: National Institutes of Health; Glu: Glucocorticoid;
MTX: Methotrexate; LEF: Leflunomide; CYS: Cyclophosphamide;
NS: Non Significant; SD: Standard Deviation; ESR, Erythrocyte
Sedimentation Rate; CRP: C-Reactive Protein.
Note: a ESR lower detection limit, 20 mm/h (females) and 15 mm/h
(males); b CRP lower detection limit, 8 mg/L; P:<0.001.
Table 1: Clinical and laboratory data of the study group (n=40).
18F-FDG -PET/CT imaging
All 40 patients had evidence of TAK based on the PET/CT findings. The PET/CT abnormalities were in the aorta abdominalis (68%), ascending aorta (68%), (63%), arteria carotis communis (53%), arteria subclavia (47%), and renal artery (21%). Four patients (11%) had involvement of the pulmonary artery, brachial artery, trunk brachiocephalic artery, and iliac artery. In addition, 8 (20%) patients had some evidence of imaging remission during clinical remission. The diseased arteries that showed improvement were the aorta abdominalis, aortic arch, renal artery, and arteria iliaca communis. For all involved arteries, the active disease and clinical remission groups had no statistically significant differences (all P>0.5).
Quantitative analysis of changes in the standardized uptake (SUV) values of 19 patients showed there were tendencies for a lower SUVmax (2.16 ± 0.46 vs. 2.08 ± 0.49, P=0.56) and a higher SUVmean (2.34 ± 0.57 vs. 2.42 ± 0.81, P=0.46) during remission (Figure 1). Only 8 (20%) patients achieved imaging remission. Overall, 27 patients (68%) had SUVmax values of 2.0 or more in visual vasculitis with positive PET/CT before treatment and the other 13 (32%) had SUVmax values below 2.0.
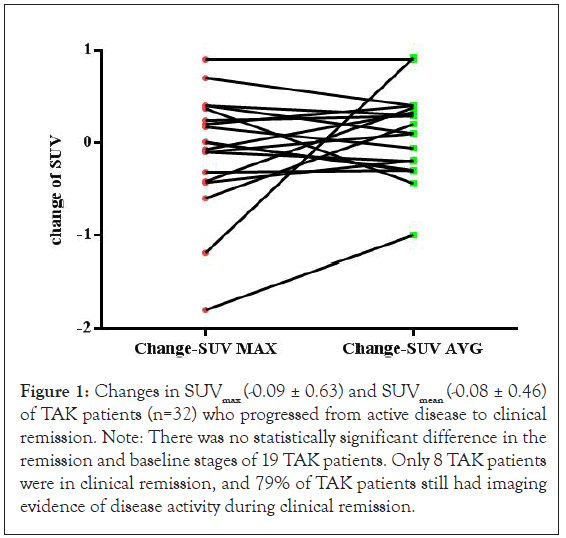
Figure 1: Changes in SUVmax (-0.09 ± 0.63) and SUVmean (-0.08 ± 0.46) of TAK patients (n=32) who progressed from active disease to clinical remission. Note: There was no statistically significant difference in the remission and baseline stages of 19 TAK patients. Only 8 TAK patients were in clinical remission, and 79% of TAK patients still had imaging evidence of disease activity during clinical remission.
Figure 2 shows PET/CT SUV changes in a representative patient during active disease and remission. In this patient, the pulmonary artery SUVmax during remission (1.94) was lower than during active disease (2.65), and the SUVmean during remission (2.01) was higher than during active disease (1.96).
Figure 2: CT images (top) and PET images (bottom) of the pulmonary artery of a representative patient with TAK who had multiple PETpositive lesions with vascular inflammation. Note: A, A’: SUVmax during active disease (January 2016) was 2.65 (SUVmean 1.96). B, B’: SUVmax during remission (October 2017) was 1.94 (SUVmean 2.02). Red arrow represents a PET-positive joint (green/yellow). SUV: standardized uptake value.
Arterial SUVmax in patients with active and inactive TAK
The maximum standardized uptake value (arterial SUVmax) was significantly higher in patients with active disease than in those with inactive disease (2.69 ± 0.50 vs. 1.843 ± 0.13, P<0.001), (Figure 3).
Figure 3: Arterial SUVmax in TAK patients with active disease (n=8) and TAK patients in clinical remission (n=32). Note: ***p<0.001.
TAK is a chronic, idiopathic, inflammatory disease that affects the great vessels (ascending aorta, pulmonary trunk, pulmonary veins, superior vena cava, and inferior vena cava) [8]. Diagnosis, treatment, and follow-up of these patients are difficult due to its non-specific clinical manifestations, the lack of specific biomarkers and characteristic imaging changes, and the lack of a unified drug regimen. Nonetheless, assessment of disease activity and evaluation of the efficacy of different treatments are essential. Some patients who have signs and symptoms of active disease have no elevation of acute phase reactants and other patients who appear to have no disease have laboratory or radiological results indicative of disease [5]. Although the NIH criteria use inflammatory markers, such as ESR and CRP, as part of the assessment, the methods currently used to assess disease activity or remission are insufficiently sensitive to detect low levels of vascular inflammation.
18F-FDG PET/CT combines functional information from PET with anatomical information from CT [26]. It is a potentially valuable technique for the diagnosis and monitoring of TAK because it can show vascular inflammation even when there are no structural changes. Importantly, 18F-FDG PET/CT is a non-invasive imaging method that measures the accumulation of 18F-FDG in vessels that have high rates of metabolism due to infiltration by inflammatory cells [27]. Inflammatory tissues have increased glucose metabolism, and this metabolism can be noninvasively measured by the uptake of radio-labeled glucose [28]. Even in patients receiving steroid therapy, it is possible to distinguish between those with active and inactive disease using 18F-FDG PET/CT [28]. In our study, TAK patients who met the NIH criteria for clinical remission nonetheless still had active subclinical vascular inflammation based on 18F-FDG PET/Ct. The PET/CT method we used is only functional, and the results only indicate the activity of vascular inflammation. Similar to the results of Grayson, et al. [29], we found that 18F-FDG PET/ CT can predict TAK recurrence and may therefore help clinicians to initiate treatments that reduce the risk of progression to more active disease.
In addition, we found no association between the type of drugs used by patients (corticosteroids, anti-TNF therapy, and immunosuppressant’s) and the severity of the inflammatory process. However, due to the limited number of cases that we examined, the safety and long-term efficacy of these drugs in patients with TAK and Coronary Artery Disease (CAD) remain uncertain. Ishikawa and Maetani examined 120 TA patients and reported the 15-year survival rate was 93% for those with prolonged remission, 68% for those with persistent active disease, and 40% for those with persistent and severe vascular disease. In addition, the mortality rate of patients with TAK and CAD together was between 3% and 21% after diagnosis [30]. These large differences in patient outcomes are due to different study methods, different follow-up times, different demographic and clinical characteristics of the patients, and use of different therapies. Our imaging study indicates that sole reliance on clinical and laboratory data may lead to bias, and suggests that routine use of 18F-FDG PET/CT imaging may improve patient outcomes.
A limitation of our study is that we only examined 32 patients who were in clinical remission. It is necessary study a larger sample of patients and to follow them for a longer time to assess the value of 18F-FDG PET/CT in predicting the prognosis of patients with TAK. An important issue is whether patients should continue treatment after achieving clinical remission of TAK, even when the imaging results indicate some persistent inflammation. The accurate assessment of disease activity is currently a challenging issue. It is very important to develop more appropriate methods for clinical assessment that can be used to guide clinical treatments, improve quality-of-life, and prolong survival. Our results suggest that vascular inflammation and injury remain present in TAK patients who are in clinical remission, and suggest that treatments should be considered for these patients.
Taken together, our results suggest that angiography alone seems inadequate for assessment of structural damage due to TAK. Other methods, such as power doppler ultrasound, MRI, and PET/CT, should be considered when clinicians want a more comprehensive and non-invasive assessment of disease activity. Our results also suggest that researchers should reconsider the current criteria used to diagnose and monitor TAK and the response to different mitigation measures. We believe that the use of clinical and laboratory data with imaging from 18F-FDG PET/CT may benefit these patients.
In summary, the criteria for TAK clinical remission are not completely consistent with the actual vascular inflammatory activity based on 18F-FDG PET/CT. Patients with TAK who have achieved clinical remission but not imaging remission have a strong need for clinicians to pay attention to their progress so as not to delay their progress. 18F-FDG PET/CT plays an important role in monitoring the imaging progress or remission of TAK patients and is an important tool for follow-up of TAK patients.
The protocol used in this study was approved by the Ethics Committee of Xijing Hospital. All included patients provided written informed consent. (Approval No. KY20163015-1).
All the patients participated voluntarily and signed informed consent.
Qing Han, Xiang Zhou, Jie Han and Jin Ding: Data curation, formal analysis, methodology, software, validation, visualization, writing the original draft, and writing and reviewing the final draft. Junfeng Jia, Kui Zhang and Fei Kang: Supervision, methodology. Qiang Liang, Zhaohui Zheng, Jing Wang and Ping Zhu: Formal analysis, investigation, methodology, supervision, validation, visualization, writing and reviewing the final draft. Zhaohui Zheng, Jing Wang and Ping Zhu: Formal analysis, investigation, methodology, supervision, validation, visualization, writing the original draft, and writing and reviewing the final draft.
Thanks to all authors and patients for their contributions.
This study was supported by the National Key Research and Development Program of China (No.2017YFC0909000). Shaanxi Province 2022 Natural Science Basic Research Program (No.2022JM-445).
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref ] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref ] [Google Scholar] [PubMed]
Citation: Han Q, Zhou X, Han J, Ding J, Kang F, Zhang K, et al. (2022) 18F-FDG-PET/CT Plays a Key Role in Formulating Treatment Strategies for Takayasu Arteritis. Angiol Open Access.10:295.
Received: 29-Nov-2022, Manuscript No. AOA-22-20512; Editor assigned: 01-Dec-2022, Pre QC No. AOA-22-20512 (PQ); Reviewed: 15-Dec-2022, QC No. AOA-22-20512; Revised: 22-Dec-2022, Manuscript No. AOA-22-20512 (R); Published: 29-Dec-2022 , DOI: 10.35248/2329-9495.22.10.295
Copyright: © 2022 Han Q, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.