Journal of Antivirals & Antiretrovirals
Open Access
ISSN: 1948-5964
+44 1300 500008
ISSN: 1948-5964
+44 1300 500008
Market Analysis - (2020)
The Rare Diseases are distributed in such a way that forty of the cases accounted by some 350 Rare Diseases. About only 5% of rare diseases are having approved drug treatment with only 326 new drugs being approved by the FDA and brought in to the market. Rare diseases are mostly often debilitating or even the life-threatening diseases or in some case the conditions which prevalence of 0.65%- 1%, cause of rare diseases is mostly genetic in disorder and sometimes it may be as a result of infections or degenerative causes.
In 2019, Orphan Drug sales were the order of 93 billion. Orphan Drugs mostly represented by 35% of the industry’s for new drug approvals. The genetic disorders are diagnosed by the area, Genetic which are going to lead the global market in the past will show similar traction in the next coming eight years. According to Statistics of MRC, the Global market of Orphan Drug is to estimate by $145.89 million in 2018 and expected to reach $265.63 million by 2022 growing at a CAGR of 10.5% from 2018 to 2022.
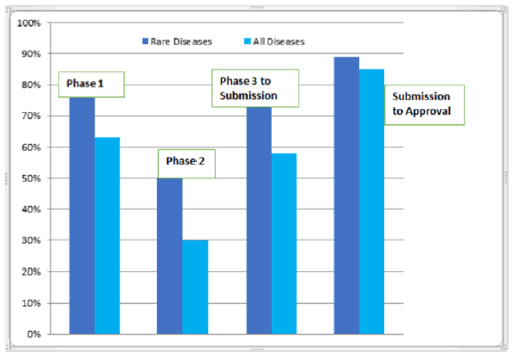
The worldwide market for rare diseases will possibly improve up at a consistent pace of 6.5% from 2017 to 2025, at this pace; the market is anticipated from US$17,318.0 Mn in 2016 to US$30,314.5 MN by the end of 2025.Estimation of the report is done by Transparency Market Research.
Rare Diseases usage increases with an increase in the wide spread of diseases and with mounting diseased population. Rare Diseases globally used for applications influencing research discovery of new drugs by academic personalities, disease diagnosis in clinical and healthcare centres.
Diseases Diagnosis which is recorded to be $11.9 billion in the year 2013 is about to reach $13.1 billion by 2019 with a developing CAGR of 1.6% between 2014 and 2019. Drug discovery is relied upon to reach $85.8 billion by 2022 from $54.7 billion by 2017 with a development in CAGR up to 9.4% from 2017 to 2022.
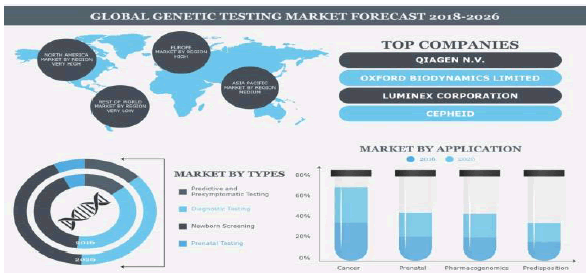
The global Rare Diseases market is driven by ease of transportation of slides, surge in prevalence of chronic diseases & aging population, and increase in the laboratory workflow efficiency. However, there is a restriction in the market growth due to lack of reimbursement and dearth of skilled personnel. Furthermore, unmet pathological needs in the emerging economies are expected to provide numerous opportunities for the market growth during the forecast period.
Key Benefits
• Along with current trends and future estimations, an in-depth analysis of the market has been studied to elucidate the imminent investment pockets.
• To enable the stakeholders to capitalize on the prevailing market opportunities, It offers a quantitative analysis of the industry from 2016 to 2023 to enable the stakeholders to capitalize on the prevailing market opportunities.
• The use of pathological product in detecting cause, origin, and nature of diseases can be elucidated by Extensive analysis of product
• To determine the prevailing opportunities, Comprehensive analysis of all geographical regions is provided.
• To understand the competitive outlook of the global market, Key players are profiled and their strategies have been analysed thoroughly.
Rare Diseases Market Overview
The global rare diseases market generated $17.4 billion revenue in 2018 and is projected to advance at a CAGR of 5.8% during the forecast period, mainly on account of increasing occurrence of chronic diseases, rising geriatric population, and advancements.
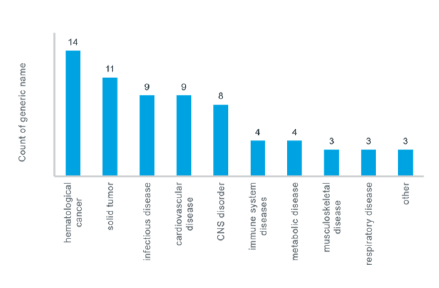
Rare Diseases Market Dynamics
Between 5,000 and 8,000 distinct rare diseases existed, affecting between 6% and 8% of the population in total – in other words, between 27 million and 36 million people in the EU. Most of the people suffer from rare diseases affecting fewer than 1 in 100,000 people.80% of rare diseases have identified genetic origins, and affect between 3% and 4% of births. Rare diseases causes due to degenerative and proliferative. Symptoms of some rare diseases may appear at birth or in childhood, including spinal muscular atrophy, lysosomes storage disorders, patent duct us arteriosus (PDA), familial adenomatous polyposis (FAP) and cystic fibrosis. Most of the rare diseases appear during adulthood, such as renalcell carcinoma and acute myeloid leukemia.
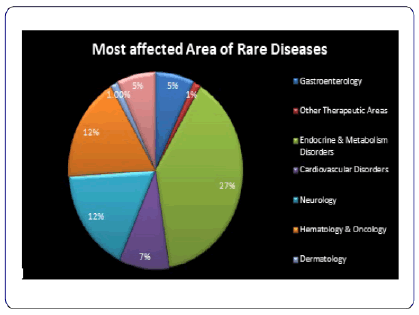
Rare Diseases and Orphan Drugs Market Competitive Landscape
On-going acquisitions and collaborations among players in the Rare Diseases and Orphan Drugs market are resulting in accurate diagnosis and increased market reach and share of the players. For instance, in December 2018, NeoGenomics Laboratories Inc. (NeoGenomics), a leading provider of cancer-focused genetic testing and information services, acquired a U.S-based Genoptix Inc., a clinical oncology laboratory, specialized in haematology and solid tumour testing. Also, in February 2019, Quest Diagnostics Incorporated, a U.S.-based provider of diagnostic information services, partnered with Houston Healthcare, a community hospital based in the U.S., to enhance the quality and value of diagnostic services through an agreement under which Quest Diagnostics Incorporated will provide full laboratory management services. The accretion is expected to expand NeoGenomics’ reach into oncology practices, and significantly accelerate the company’s progress toward key scale and growth objectives.
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.