Review Article - (2016) Volume 2, Issue 4
A Brief Overview on Recent Advances in the Development of Anti-Tubercular Compounds Containing Different Heterocyclic Ring Systems
*Corresponding Author: Mohammad Asif, Department of Pharmacy, GRD (PG) Institute of Management and Technology, Dehradun, Uttarakhand, India, Tel: +91 92580-71905, Fax: +91 135 273-4048 Email:
Abstract
Tuberculosis (TB), a leading cause of mortality and morbidity with more than one-third of the world population infected with latent TB and the worldwide dissemination of multidrug (MDR) and extensively drug resistant (XDR) Mycobacterium tuberculosis poses a serious threat to human health. Hence, new drugs are urgently needed to shorten and improve the treatment course in drug resistant TB, and to minimize the occurrence of new infections and death to zero level. Various new drugs progress to be developed for the treatment of MDR-TB. Several new molecules in clinical development encourage the scientific community to find new drug targets and new drug leads. In this perspective we present herein an overview of the new anti-TB agents with different molecular structures. Here we have tried to provide some efforts that are being made in the development of new drug molecules as lead anti- TB agents.
Keywords: Tuberculosis; Mycobacterium tuberculosis; Multidrug resistance; Extensively drug resistance; New drugs and targets
Introduction
Heterocyclic chemistry is the branch of chemistry dealing with synthesis, properties, and applications of heterocycles. Heterocycles form by far the largest of the classical divisions of organic chemistry and are of immense importance biologically, industrially, and indeed to the functioning of any developed human society. The majority of pharmaceuticals and biologically active agrochemicals are heterocyclic. There are countless heterocyclic additives and modifiers used in industries [1-3]. Heterocycles play an important role in biochemical processes. Heterocyclic systems occur in a wide variety of natural and synthetic compounds and are essential to life in various ways. The synthetic heterocyclic drugs are still more numerous and include most of the antimicrobials, hypnotics, anti-convulsants, analeptics, antihistaminics anti-thyroid drugs, also many antiseptics, fungicides, vasopressor modifiers and others. Heterocyclic rings constitute a large number of synthetic dyes and analytical reagents [3-6].
Tuberculosis, commonly known as TB, is an often severe and contagious airborne disease caused Mycobacterium tuberculosis (Mtb ) and typically affects the lungs but can affect the other parts of the body called extrapulmonary tuberculosis (TB). The Mtb is acid-fast, gram positive bacteria, grows slowly under aerobic conditions. Multidrug- Resistant TB (MDR-TB) is defined by resistance to the two most commonly used drugs in the current four-drug (or first-line) regimen, isoniazid and rifampin. Extensively drug resistance TB (XDR-TB) is caused by Mtb resistant to isoniazid, rifampin, at least one fluoroquinolone, and one of the injectable anti-TB drugs such as amikacin, kanamycin, or capreomycin. Minimum Inhibitory Concentration (MIC) is the concentration of antibacterial that will inhibit the growth of bacteria. DOTs (Directly Observed Treatment, Shortcourse) is a strategy that framework for the TB control programme [7-10].
TB is one of humanity’s oldest and most resilient plagues, despite the availability of four drug regimen to treat the disease [11]. The current first line anti-TB regimens require a minimum 6 months of DOTs therapy. Adherence to the long and complicated treatment course is challenging and is a major obstacle to the effective use of existing drugs [12]. As a result of treatment failure and poor observance, epidemic with MDR-TB or XDR-TB is being more common [13]. In 2011, the number of MDR-TB infections was estimated at 60,000 cases (19 % of the global infected population) [4]. Suggested regimens for MDR-TB therapy require at least 20 months of treatment with drugs that are toxic, poorly tolerated, and limited efficacy of cure rate. According to World Health Organization (WHO) global TB report 2012, there were almost 9 million new cases in 2011 and 1.4 million TB deaths. Besides, the emergence of drug-resistance is becoming a major threat to global TB care and control. Around 310,000 MDR-TB cases occurred among notified TB patients in 2011 [14]. The increasing emergence of DR-TB and HIV infection which compromises host defense and allows latent infection to reactivate TB and posed further challenges for effective control of TB. Moreover, TB treatment is lengthy (takes 6-9 months) with significant toxicity, which creates poor patient compliance resulting in a frequent cause for selection of drug resistant and often deadly MDR-TB bacteria [15]. In 2013, 6.1 million TB cases were reported out of these, 5.7 million were newly diagnosed. Number of MDR-TB infections was estimated at 23% of reported TB patients. 1.1 million (13%) of the 9 million people who developed TB in 2013 were HIV-positive. About 60% of TB cases and deaths occur among men and 510000 women died as a result of TB, more than one third of whom were HIV-positive. There were 80000 deaths from TB among HIV negative children in the same year [16]. The emergence of highly lethal, expensive and virtually untreatable XDR-TB poses a new threat to TB control. The control of TB is complicated due to latent TB where the infected persons are asymptomatic, and serve as the reservoir for the pathogen, making control of this disease a difficult and challenging task [17]. In 2014, the WHO estimated 9 million new TB cases had occurred globally in 2013,480000 of them being affected by MDR-TB strains [18]. The MDR-TB treatment success is only 54% (with 15% death, 8% failure/relapse and 23% default). When the drug resistance profile is beyond XDR (with increasing complexity), the outcomes are unfortunately lower: treatment success ranges from 40% to 19%, failure/relapse from 15% to 54% and death from 15% to 35% [19,20]. Every day, clinicians managing these cases face relevant challenges that include frequent occurrence of adverse events, problems in patients’ adherence, lack of clinical experience, and limited availability of adequate diagnostics and second-line anti-TB drugs. The risk of acquiring further drug resistance is therefore real. WHO has launched its innovative “End TB Strategy”, supporting the TB elimination strategy and the vision of a TB-free world with zero death, disease and suffering due to TB [21-23]. The strategy clearly supports universal access to high-quality MDR-TB diagnosis and treatment [24]. The need for new drugs and regimens is obvious [25].
Recent advances in the knowledge of molecular biology and Mtb genome sequences has enabled the essentiality of genes for the rapid target identification for the new anti-TB agents via identification of mutated genes of compound-resistant mutants [26,27]. Effective treatment of TB patients co-infected with HIV is complicated due to drug-drug interactions between anti-retrovirals (ARVs) and antituberculosis drugs and increased the risk of adverse effects. There is urgent need for more effective and tolerable anti-tuberculosis therapy for the treatment of drug-susceptible, drug-resistant disease and latent-TB infection [28]. Regimens that can be safely coadministered with antiretroviral therapy are urgently needed for the growing number of patients co-infected with both HIV and TB. These approaches include increased funding for research in antibiotic resistance and drug development for TB, development of methods for protecting the efficacy of existing drugs, and prioritization for making use of current non-TB drugs for TB treatment [29]. Among MDR-TB patients started on treatment globally in 2009, only 48% were treated successfully, largely as a result of a high frequency of patient deaths (15%) and loss to follow-up (28%), which is commonly associated with adverse drug reactions, among other factors. New drugs that would help build a better, safer, less toxic, shorter and cheaper regimen are therefore urgently needed to reduce patient suffering and mortality [30]. It has been over 40 years since a new drug for tuberculosis has been discovered [31]. Therefore, the development of innovative, effective drug combinations should also be encouraged to diversify therapeutic choices, especially those for drug resistant TB cases [29].
Designing a regimen to treat TB
The treatment regimens approved TB drugs and the dosage of anti- TB drugs recommended by the evidence-based WHO guidelines. “New” and “retreatment” cases are clearly separated, 30 days of previous anti-TB treatment being the cut-off. New TB cases (irrespective of HIV status) should be treated for the first 2 months (intensive phase) with isoniazid, rifampicin, pyrazinamide and ethambutol, followed by isoniazid and rifampicin for the remaining 4 months (continuation phase) [32]. The daily dosage is recommended (although the three times weekly dosing can be used during the continuation phase under directly observed therapy) as well as the fixed-dose combinations [33]. The aim of this review is to summarise some anti-TB compounds.
Pyrimidines, dihydropyrimidines, tetrahydropyrimidines
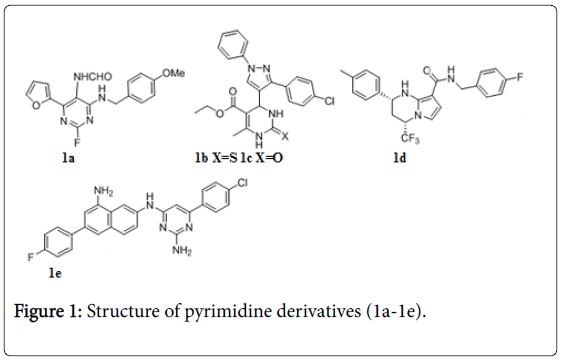
Various pyrimidine analogs (Figure 1) were tested against Mtb [34-38]. Compound 1a (5-formamidopyrimidines) displayed IC90 values ≤ 1 μg/mL, and exhibited low toxicity towards mammalian cells. A series of dihydropyrimidines also exhibited in vitro anti-TB activity against Mtb H37Rv, Compounds 1b, 1c were found to be the potent against Mtb with MIC value 0.125 and 0.25 μg/mL respectively [39]. Tetrahydropyrazolopyrimidine, 1 d exhibited in vitro MIC value 0.15 ± 0.04 μM and potent in vivo activity in a mouse efficacy model, achieving a reduction of 3.5 log CFU of Mtb after oral administration to infected mice once a day at 100 mg/kg for 28 days [40]. One of the quinolinyl pyrimidines 1e showed MIC0.87 μg/mL and enzyme inhibition (IC50=0.043 μM) against the NDH-2 target, which in turn translated into cellular activity against Mtb [41].
Piperidine-4-ones
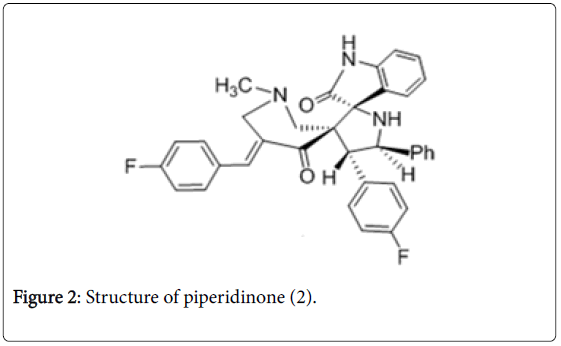
Piperidinone derivatives were reported as potent anti-TB agents [42-45]. The 4-(4-Fluorophenyl)-5-phenylpyrrolo(spiro[2.3′ ′]oxindole)spiro[3.3′]-1′-methyl-5′-(4-fluoro-phenyl methylidene) piperidin-4′-one (Figure 2) was found to be active in vitro with a MIC value of 0.07 μM against Mtb . In vivo , compound 2 decreased the bacterial load in lung and spleen tissues with 1.30 and 3.73-log 10 protections respectively and was considered to be promising in reducing bacterial count in lung and spleen tissues.
Quinoxaline 1,4-dioxides
The leading compound LVTZ 3a (Figure 3) belongs to quinoxaline 1,4-dioxides class of compounds showed very good selectivity and activity against Mtb with MIC 0.1 μg/mL [46]. Anti-TB screening of 3- methyl-2-phenylthioquinoxaline 1,4-dioxides (3b-3f) exhibited MIC between 0.39 and 0.78 μg/mL against Mtb . Amide of quinoxaline 1,4- di-Noxides 3g were active against Mtb as same as rifampin (RIF) [47]. A series of quinoxaline derivatives exhibited promising anti-TB activity compound 3h of them emerged as a lead compound having IC50 and IC90 figures of 1.03 mM and 1.53 mM, respectively by affecting the respiration in rat liver mitochondria [48]. New lead compound 3i from Benzotriazine Di-N-Oxides series has MIC 0.31 μg/mL against H37Rv and cytotoxicity (CC50) against Vero cells of 25 μg/mL. This was also negative in a L5178Y MOLY assay, indicating low potential for genetic toxicity [49].
Diydropyridines
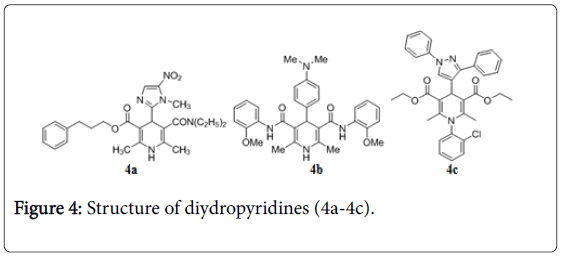
1,4-Dihydropyridines are the emerging class of anti-TB agent [50,51]. Compound 4a (Figure 4) exhibits anti-TB activity with MIC 1 μM, in vitro screening [52]. 3D-QSAR study reveals new derivative of 1,4-dihydropyridines compound 4b with anti-TB activity [53]. Compound 4c was evaluated as potent antitubercular compound having MIC 0.02 μg/mL and low toxicity [53].
Imidazolopyridines and pyrazolotetraydropyridine
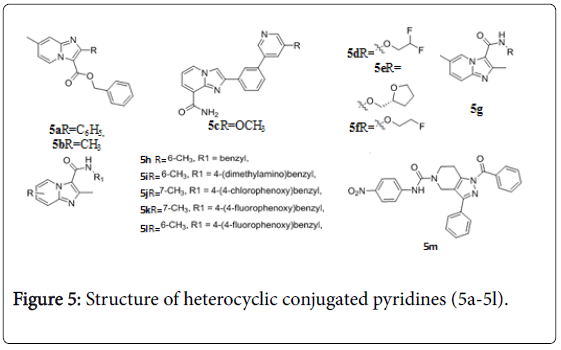
Imidazopyridines were determined to have promising anti-TB activity against replicating Mtb H37Rv, compounds 5a and 5b (Figure 5) exhibited MIC value< 0.195 μM [54]. Anti-TB activity of imidazopyridine- 8-carboxamides (Figure 5) were evaluated, compounds 5c-5f exhibited MIC value 0.5, 0.5, 0.25, and 0.25 μg/mL against M. tuberculosis [55]. A series of 2,7-dimethylimidazo[1,2-a] pyridine-3- carboxamides 5g were evaluated for their in vitro anti-TB activity versus replicating, nonreplicating, multi- and extensive drug resistant Mtb strains. The MIC90 values of these compounds were < 1 μM against the various TB strains tested [56]. The MICs of compounds (5h-5l) against replicating bacteria had MIC values ≤ 0.006 μM. These results indicate that readily synthesized imidazo[1,2-a]pyridine-3- carboxamides (figure 5) are an exciting new class of potent anti-TB agents that merit additional development opportunities [57]. 1- benzoyl-N-(4-nitrophenyl)-3-phenyl-6,7-dihydro-1H-pyrazolo[4,3- c]pyridine-5(4H)-carboxamide (5m) was found to be active with IC50 of 21.8 ± 0.8 μM against Mtb PS [58].
Galactopyranosyl amino alcohols
A dimeric hybrid of a galactopyranosyl amino alcohol 6 displayed potent in vitro activity with MIC 1.56 μg/mL against Mtb . However, on progression into a murine model, toxicity was observed at dosage levels (50 mg/kg per day) that offered no significant protection against Mtb infection (Figure 6). The target of this compound is mycobacterial cell wall biosynthesis [59].
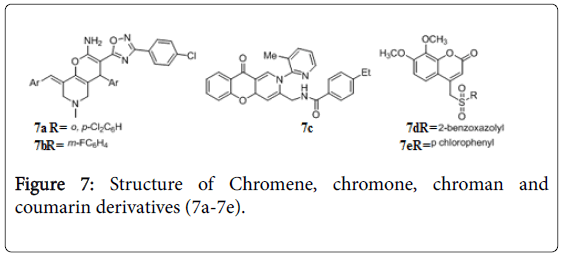
Chromene, chromone, chroman and coumarin derivatives
The chromene, chromane and its analogue are reported to have anti-TB activity [60,61]. Oxadiazole-chromenes 7a, 7b (Figure 7) exhibited in vitro activity with MIC 0.31 μg/ mL and 0.73 μg/mL against Mtb H37Rv. Recently 2,10-dihydro-4aH-chromeno[3,2-c] pyridin-3-yl derivatives were evaluated for their activity against Mtb H37Rv and MDR-TB. Among them compound 7c was found to be active in vitro with MIC’s of 0.22 and 0.07 μg/mL against Mtb and MDR-TB respectively. During the in vivo study in animal model compound 7c decreased the bacterial load in lung and spleen tissues with 1.11 and 2.94 log10 protections at 25 mg/kg body wt. dose [62]. Arylsulfonyl-methylcoumarin screened for in vitro anti-tubercular activity against Mtb H37Rv, compounds 7d and 7e showed MIC 0.78 μg/mL and 1.56 μg/mL respectively [63]. Phenyl substituted coumarins [64], and spirocromone conjugates [65] also displayed potent activity against TB.
Thiazoline, thiazole, benzothiazinone and dithiazolone analogues
The anti-TB activity in thiazoline class of compounds (Figure 8) has been reported recently [66]. The most potent compound 8a of this series showed MIC 0.3 μg/mL. A series of potent 5-(2 methylbenzothiazol-5-yloxymethyl) isoxazole-3-carboxamide derivative 8b, led to potent anti-TB activity with MIC value 1.4 μM against replicating Mtb H37Rv [67]. Several other thiazoles and benzothiazoles are reported as potent inhibitor of Mtb [68-70]. A series of benzothiazinones, 8c-8e of this series showed MIC ≤ 0.015 μg/mL activities against MDR-TB with low toxicity [71]. Heterocycle substituted 1,3-benzothiazin-4-one derivative 8f showed MIC of 0.0001 μM against Mtb H37Rv, 20-fold more potent than BTZ043 racemate [72,73]. Compound 8g dithiazol-3-one derivative was found to be active with a lowest MIC90 value of 1 μg/mL [74].
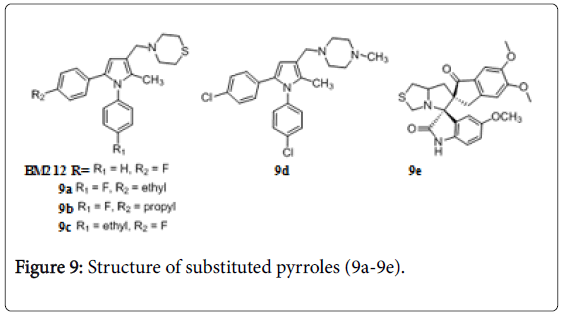
Pyrrole and pyrrolotiazole
Pyrrole derivative BM 212 is moderately active against Mtb (MIC=0.7 to 6.2 μg/mL) and M. avium (MIC 0.4 to 3.1 μg/mL) [75]. The thiomorpholine introduction in BM 212 molecule improved its anti-TB activity. Four compounds 9a, 9b, 9c and 9d (Figure 9) had MIC between 1 and 2 μg/mL [76,77]. Several derivatives have shown significant activities against drug-resistant TB in vitro and offer considerable protection in a rigorous mouse model of the disease [78]. Dispiropyrrolothiazoles derivative 9e showed anti-TB activity against Mtb H37Rv and INH resistant Mtb strains with MIC of 0.210 and 8.312 μM respectively [79].
Oxazole, Oxadiazole and Isoxazoline derivatives
Several 2-(biphenyl-4-yl)oxazole-4-carboxylates possess good activity against Mtb with extremely low toxicity toward VERO cells and high therapeutic indexes [80]. Oxadiazoles 10a and 10b (Figure 10) indicate inhibition of Mtb at concentrations 1.6 and 1.5 μg/mL [81]. Compound 10c showed in vitro anti-TB activity with MIC value 0.07 and 0.14 mM against Mtb and MDR-TB respectively [82]. Several oxazoles [83] and oxadiazoles are identified as potential candidate for the treatment of MDR and XDR tuberculosis [84,85]. Nicolas Willand reported thiophen-2-yl-1,2,4-oxadiazoles 10d, 10e as EthR inhibitors that boost antibacterial activity of ethionamide with nanomolar potency [86]. The anti-TB activity of isoxazoline linked nitrofurans compounds 10f-10j was reported [87]. Very good in vivo efficacy in analogue of phenylisoxazoline 10k with MIC as low as 0.5 μg/mL [88].
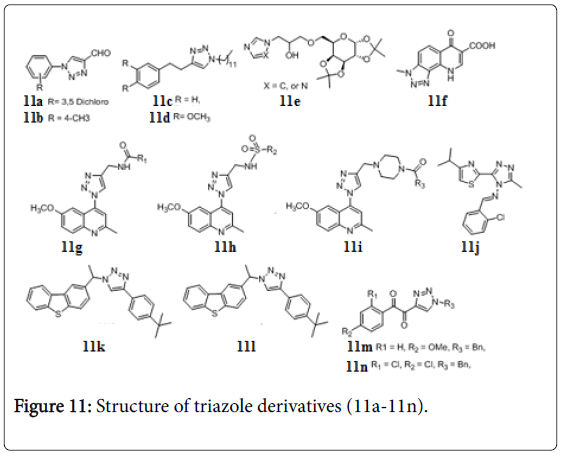
Triazoles
Several triazoles were evaluated for their anti-TB activity against Mtb H37Rv (MIC 3.12-12.5 μg/mL) [89,90]. N-substitutedphenyl- 1,2,3-triazole-4-carbaldehydes 11a and 11b (Figure 11) showed inhibition at MIC 2.50 μg/mL [91]. The evaluated triazoles as inhibitors of InhA as well as inhibitors of Mtb H37Rv. Compound 11c and 11d (Figure 11), were good inhibitors against Mtb with MIC 0.50 and 0.25 mg/mL, respectively [92]. Preliminary results of galactoselinked triazoles, exhibited MIC values in the range of 1.56-12.5 μg/mL against Mtb H37Rv. Compound 11e inhibited bacterial growth at MIC 1.56 μg/mL [93]. A number of triazole and quinolone hybrids have been reported to possess anti-TB activity, compound 11f showed MIC 0.5 μg/mL against Mtb [94]. Three new series of quinoline-4-yl-1,2,3- triazoles carrying amides 11g, sulphonamides 11h and amidopiperazines 11i possess MIC 1 μg/mL against Mtb H37Rv [95]. 2-substituted-5-[isopropylthiazole] clubbed 1,2,4-triazole 11j, exhibited promising activities against Mtb H37Rv strain [84]. 1,2,3- triazolebased Mtb inhibitors and tricyclic (carbazole, dibenzo[b,d] furan, and dibenzo[b,d]thiophene) were integrated in one molecular platform to prepare various novel clubbed 1,2,3-triazole hybrids as potential inhibitors of Mtb H37Rv. Two of them 11k and 11l inhibit the Mtb at MIC 0.78 μg/mL [96]. α-ketotriazole and α,β-diketotriazole derivatives were evaluated for anti-TB and cytotoxic activities. Among them, two α,β-diketotriazole compounds, 11m and 11n, exhibited good activities (MIC=2.5 μg/mL) against Mtb and MDR-TB strains and presented no cytotoxicity (IC50>50 mM) on colorectal cancer HCT116 and normal fibroblast GM637H cell lines [97].
Imizazoles, pyrazoles and pyrazolones
Several nitroimidazoles were reported as potent anti-TB agents [98]. MIC of 12a turned out to be 0.5 μg/mL and compound 12b showed activity as good as PA-824 against non-replicating Mtb [99]. New class of 2-(trifluoromethyl)-6-arylimidazo[2,1,b][1,3,4] thiadiazole derivative 12c has MIC 1.56 μg/mL against Mtb H37Rv. Most compounds from the series exhibited activity within range of MIC 3.12-1.56 μg/ml [100]. Ring substituted imidazoles are the emerging class of anti-TB agents [101-104]. A series of 3-(4-chlorophenyl)-4- substituted pyrazoles were tested for anti-TB activity in vitro against Mtb H37Rv strain using the BACTEC 460 radiometric system, 2- azetidinones and 4-thiazolidinones bearing a core pyrazole scaffold, 12d-12n (Figure 12) exhibited MIC 0.85, 0.37, 0.55, 0.36, 0.6, 0.5, 0.36, 0.55, 0.65, 0.65 and 0.39 μg/mL respectively against Mtb [105]. Different analogues of 1,5-dimethyl-2-phenyl-4-([5-(arylamino)-1,3,4- oxadiazol-2-yl]methylamino)-1,2-dihydro-3H-pyrazol-3-one was also found active against Mtb H37Rv and isoniazid resistant Mtb [106]. 4- [(2,4-dichlorophenyl)(2-hydroxy-1-naphthyl) methyl]-2-(4- fluorophenyl)-5-methyl-2,3-dihydro-1H-3-pyrazolone displayed the maximum potency with a MIC of 1.6 μM against Mtb [107].
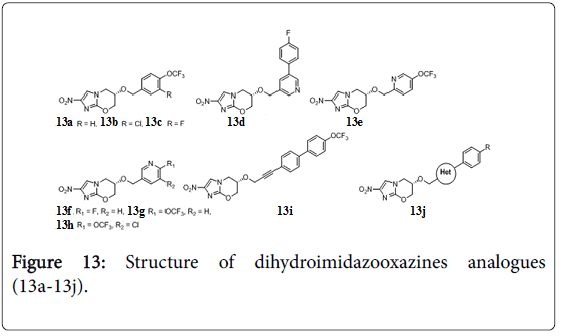
Dihydroimidazo-oxazines analogues
Biphenyl analogues of PA-824 were evaluated for their efficacy in a mouse model of acute Mtb infection. Three compounds 13a, 13b, 13c (Figure 13) bearing combinations of lipophilic, electron-withdrawing groups achieved >200-fold higher efficacies than the parent drug [108]. Heterocyclic analogues of PA-824 compounds 13d, 13e, 13f, 13g, 13h (MIC 0.31, 0.065, 0.06, 0.05, 0.017 μg/mL respectively) were >100-fold better than PA-824 in a mouse model of acute Mtb infection, and two orally bioavailable were superior to anti-TB drug OPC-67683 in a chronic infection model [109]. Different analogues of PA-824 were prepared by replacing OCH2 with amine, [110] amide, carbamates and urea functionality and investigated their improved efficacy against Mtb [111]. Extension of OCH2 linkers (propenyloxy, propynyloxy, and pentynyloxy) provided greater potencies against replicating Mtb . One propynyloxy-linked compound 13i displayed 89-fold higher efficacy than PA-824 in the acute model [112]. 1-Methylpyrazole, 1,3-linkedpyrazole, 2,4-linked-triazole, and tetrazole bearing compound 13j, analogues of PA-824 had 3- to 7-fold higher MIC potencies than parent molecule against replicating Mtb [113].
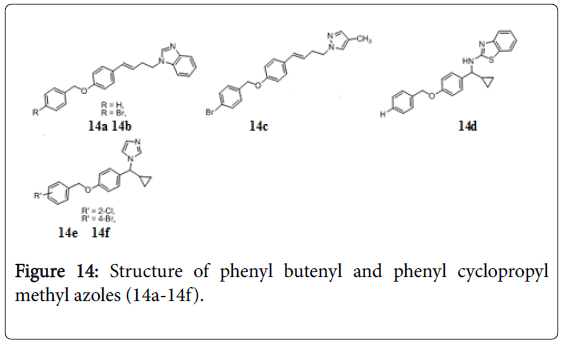
Phenyl butenyl and phenyl cyclopropyl methyl azoles
A series of 1-[(4-benzyloxyphenyl)-but-3-enyl]-1H-azoles has been identified as potent antitubercular agents against Mtb . Compounds 14a, 14b, and 14c (Figure 14) exhibited significant antitubercular activities with MIC value as low as 1.56, 1.56, and 0.61 μg/mL, respectively. Cyclopropyl methyl azoles, 14d-14f inhibited the bacterial growth at MIC 2.41, 3.12 and 3.12 μg/mL [114].
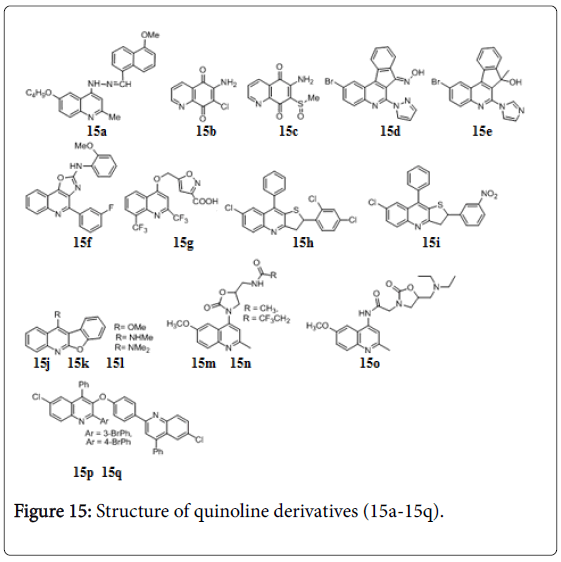
Quinolines
Several quinoline derivatives were reported with significant anti-TB activity [115-121]. 4-Quinolylhydrazone 15a the structural hybrids of isoniazid and quinolones (Figure 15) showed anti-TB activity with MIC 0.78 μg/mL but poor selectivity for mycobacteria. Several quinolinequinone, 6-amino-7-chloro-5,8-quinolinequinone 15b and 6- amino-7-methane sulfinyl-5,8-quinolinequinone 15c (Figure 15): Structure of quinoline derivatives exhibited MIC’s (1.56 and 3.13 μg/mL) for the 100% growth inhibition of M. bovis BCG [122]. The efficacies of indeno [2,1-c] quinolines were evaluated in vitro using the BACTEC radiometric assay and compounds shows 85-99% growth inhibition of Mtb . Compounds 15d and 15e (Figure 15) showed MIC, 0.39 and 0.78 μg/mL respectively [123]. Fused oxazoloquinoline 15f exhibited 99% bacterial growth inhibition and MIC, 1 μg/mL against Mtb H37Rv [124]. Another hybrid of isooxazole and quinoline 15 g is reported to have excellent anti-TB activity against both replicating and non-replicating Mtb , with MIC 0.9 μM [125]. A series of quinoline derivatives viz. hydrazones, ureas, thioureas and pyrazoles were evaluated for their Mtb H37Rv and MDR-TB [126,127]. The lead compound 2,9-diaryl-2,3-dihydrothieno[3,2-b]quinolines (15h and 15i) displayed MIC 0.90 and 0.95 μM against Mtb and MDR-TB [128]. A series of 11-alkoxylated and 11-aminated benzofuro[2,3-b]quinoline derivatives 15j, 15k and 15l (Figure 15) exhibited significant activities against the growth of Mtb (MIC values of < 0.20 μg/mL) and low cytotoxicities against VERO cell with IC50 values of 11.77, 5.55, and >30.00 μg/mL respectively [129]. Compounds 15m, 15n and 15o have MIC 0.65 μg/mL against M. tuberculosis H37Rv strain [130,131]. Phenoxy linked bisquinoline derivatives 15p and 15q have MIC 1.1 and 2.2 μM respectively against Mtb and no in vivo cytotoxic effects against mouse fibroblasts (NIH 3T3) [132].
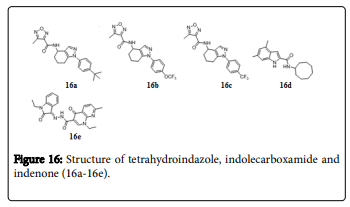
Tetrahydroindazole, Indolecarboxamide and indenone derivatives
A class of tetrahydroindazole (Figure 16) based compounds are reported as potent and unique inhibitors of Mtb . Compounds 16a, 16b and 16c exhibited MICs of 1.7, 1.9, and 1.9 μM respectively against Mtb [133]. Indole-2-carboxamide analogue, 16d showed potent antitubercular activities against actively replicating Mtb , with MIC values 0.013 μM. Compound 16e was found to be active against the tested XDR-TB strains and orally active in the serum inhibition titration assay [134]. A series of 2-(arylmethylene)-2,3-dihydro-1Hinden- 1-ones were screened for their in vitro activity against Mtb H37Rv, Compound 16f displayed MIC at 2.8 μM against Mtb [135]. A library of trans 6-methoxy-1,1-dimethyl-2-phenyl-3-aryl-2,3- dihydro-1Hinden-4-yloxy alkyl amines exhibited MIC between 1.56 and 6.25 μg/mL against drug sensitive and multidrug resistant strains of Mtb [136].
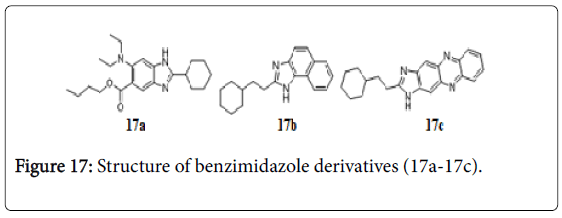
Benzimidazoles
Libraries of trisubstituted benzimidazoles were created through rational drug design. A number of benzimidazoles exhibited promising MIC values in the range of 0.5-6 μg/mL, against Mtb H37Rv strain (one of them compound 17a, has MIC 0.5 μM) (Figure 17) [137]. Compounds 17b and 17c bearing benzimidazole ring showed the potent tuberculostatic activity against Mtb with MIC of 1.56 and 3.1 μg/mL [138].
Nitrofuran and benzofuran
Several 4-(5-nitro furan-2-yl) prop-2-en-1-one derivatives, exhibited anti-TB activity against Mtb H37Rv with MIC< 5 μg/mL and low toxicity. Compound 18a (Figure 18) was evaluated as potent anti- TB with MIC 0.19 μg/mL and selective index MIC99/CC55>1800 [139]. A class of benzofuro-oxazins, 1-(4-chlorophenyl)-1H-benzo[2,3] benzofuro[4,5-e][1,3] oxazin-3(2H)-one 18b and 1-(4- bromophenyl)-1H-benzo[2,3] benzofuro [4,5-e][1,3] oxazin-3(2H)- one 18c (Figure 18) displayed same MIC 1.56 μg/mL against Mtb [140].
Triazolophthalazine and 3-aracylphthalide derivatives
Compound 19a, 4-isopentenyloxycinnamyl triazolophthalazine derivative, was found to be 100-1800 times more active than Isoniazid (INH) when tested for its ability to inhibit the growth of INH-resistant Mtb strains. It does not interfere with mycolic acid biosynthesis, thereby pointing to a different mode of action and representing an attractive lead compound for the development of new anti-TB agents [141]. 3-Aracylphthalides (Figure 19) were synthesized and were subjected to in vitro anti-TB screening against Mtb H37Ra. Among the phthalides 19b, 19c, 19d and 19e exhibited IC50 in the range of 0.97, 0.93, 0.81 and 1.24 μg/mL respectively [142].
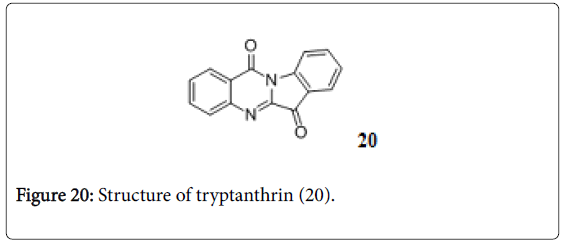
Tryptanthrin
Trypthanthrin is indolo-quinazolinone alkaloid (Figure 20) and active against MDR-TB with MIC 0.5-1.0 μg/mL. in vitro toxicity and in vivo studies are needed before this structural prototype is applied as anti-TB [143].
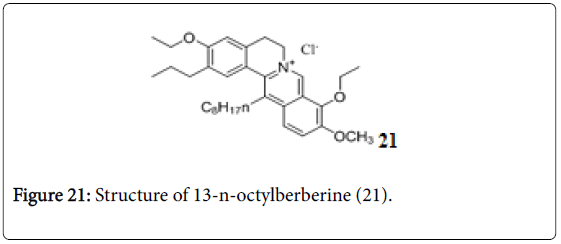
13-n-Octylberberine derivatives
A series of 13-n-octylberberine derivatives were evaluated for their anti-TB activity. Among these, compound 21 (Figure 21) was the most effective anti-TB with a MIC value of 0.125 μg/mL, and also exhibited more potent effect against rifampicin (RIF)- and isoniazid (INH)- resistant Mtb strains than both RIF and INH, suggesting a new mechanism of action [144].
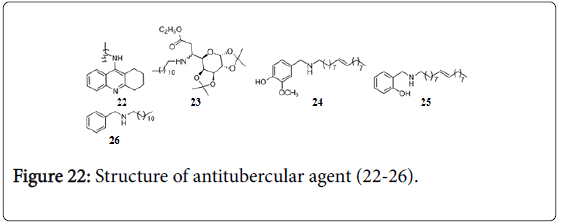
In our group a series of 9-substituted tetrahydroacridines were synthesized and evaluated against Mtb H37Rv and H37Ra strains, which exhibited potent activities with MIC 6.25-0.78 μg/mL. Comp 22 (Figure 22) was found to be most active (MIC 0.78 μg/mL against Mtb H37 Rv) [145].
Glycosyl β-amino esters [146] and glysylated amino-alcohols [147] were evaluated for their anti-TB activity against Mtb H37Ra and H37Rv. Compound 23 showed MIC 3.12 μg/ mL against both Mtb H37 Rv and H37Ra strains. Benzyl- and pyridylmethyl amines, compound 24, 25 and 26 exhibited MIC 1.56 μg/mL against Mtb . Some of them were also evaluated against clinical isolates of MDR-TB and found to be active with MIC 3.12 μg/mL [148]. α,α’-(EE)-bis(benzylidene)- cycloalkanones displayed moderate anti-TB activity with MIC 12.5-1.56 μg/mL [149]. The potent in vitro and moderate in vivo anti- TB activities thiadiazine thiones have been reported against M. tuberculosis H37Rv even in resistant strains and also protected mice marginally in experimental TB [150]. 6-Oxo and 6-thio analogue of purin [151] and carboxylic uracil derivatives [152] showed good inhibitory activity against Mtb . 4-Oxo-4-chlorophenyl-butenoyl methyl ester has MIC of 0.6 and 1.5 μg/mL against replicating and non-replicating M. tuberculosis , respectively, it penetrates the cell where it is hydrolyzed and reacts with CoA to generate the active antibacterial [153].
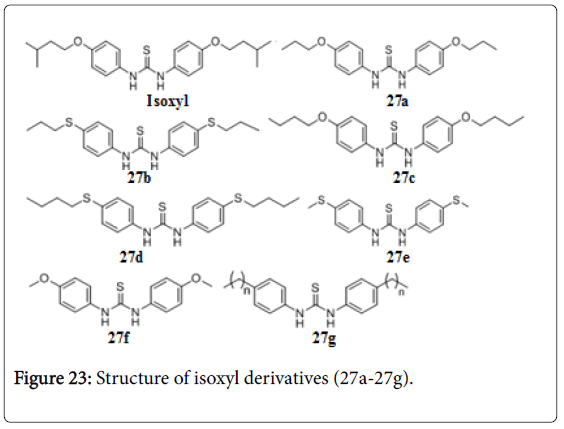
Isoxyl and urea derivatives
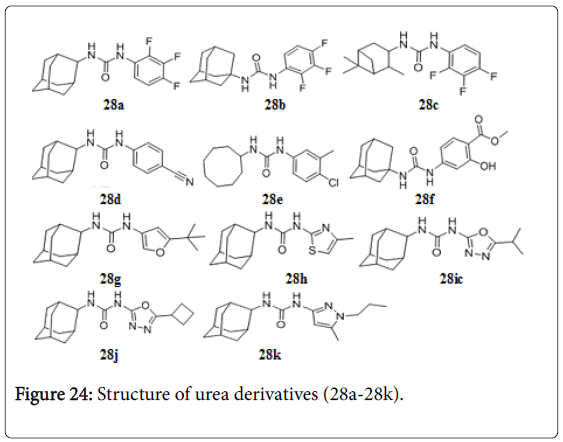
Isoxyl (ISO), thiourea (thiocarlide, 4,4’- diisoamyloxythiocarbanilide), exhibited potent activity against Mtb H37Rv (MIC, 2.5 mg/mL), M. bovis BCG (MIC, 0.5 mg/mL), M. avium (MIC, 2.0 mg/mL), and M.aurum A+ (MIC, 2.0 mg/mL), by inhibiting the mycolic acid synthesis. A comparison with isoniazid (INH) and ethionamide (ETH) demonstrated marked similarity in action. Isoxyl derivatives (27a-27g) also exhibited MIC value in the range of 0.1-0.5 μg/mL [154]. The Fas II synthesis is in involved in ISO resistance [155]. A series of 1-adamantyl-3-phenyl urea 28a-28f that had potent anti-tuberculosis activity with MIC values 0.01, 0.4, 0.02, 0.4, .01, and 0.4 μg/mL. But they had undesirable properties, particularly high lipophilicity and poor solubility [156]. A new series of 1-adamantyl-3-heteroaryl ureas 28g-28k by replacing the phenyl substituent of the original series with pyridines, pyrimidines, triazines, oxazoles, isoxazoles, oxadiazoles and pyrazoles (Figure 23). The lead isoxazole (28g, MIC 0.10 μg/mL), thiazole (28h , MIC, 1.56 μg/mL, oxadiazole (28i and 28j, MIC 1.56 and 0.78 μg/mL) and pyrazole (28k, MIC 1.56 μg/mL) substituted adamantyl ureas with improved in vitro PK profiles, increased selectivity and good anti-TB potencies (Figure 24) [157].
Hydrazines, hydrazones, thiosemicarbazone and thiocyanate derivatives
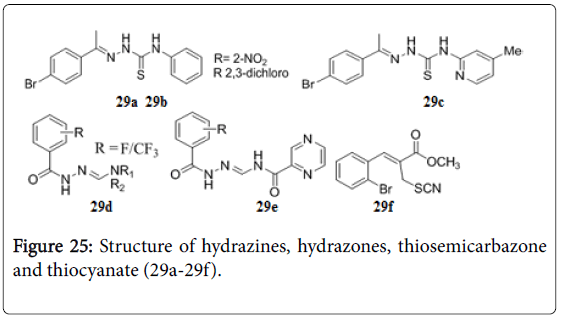
Hydrazine carbothioamides 29a, 29b and 29c were reported to have MIC 0.4 μg/mL against Mtb [158]. Fluorinecontaining hydrazones 29d and 29e (Figure 25) have shown a remarkable activity against MDRTB strain with MIC 0.5 mg/mL and high value of selectivity index [159]. 2-Bromophenyl substituted thiocyanate 29f showed MIC (0.25 μM against replicating Mtb and 8.0 μM against non-replicating Mtb ) and IC50 32 μM in the VERO cellular toxicity assay [160]. Several other hydrazones possessed anti-TB activity [161-163]. 5-nitro-thiazolylthiosemicarbazones, N-(5-nitro-1,3-thiazol-2-yl)-2-((Z)-4- [(phenylmethyl)oxy] phenyl-methylidene) hydrazine-1-carbothioamide was found to be active with a MIC of 0. 23 μM against Mtb H37 Rv, and was three times more potent than isoniazid and equally active as rifampicin [164].
Alkyl-sulfinyl amides, fatty acid amides and nitro propionamides
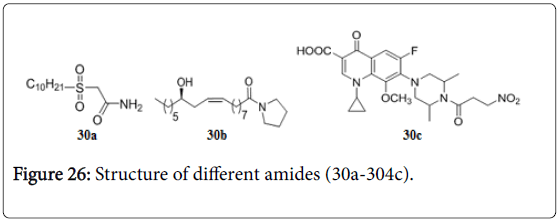
Alkyl sulfinyl amides inhibit β-ketoacyl synthase (KAS), one of the accessory fatty acid synthases peculiar to mycobacteria. The compound 30a showed good MIC at 0.75 μg/mL [165]. The fatty acid amide derived from ricinoleic acid 30b (Figure 26) is the potent one among a series of tested compounds, with MIC 6.25 μg/mL for resistance strains of Mtb [166]. 1-cyclopropyl-7-(3,5-dimethyl-4-(3- nitropropanoyl)piperazin-1-yl)-6-fluoro-8-methoxy-4-oxo-1,4- dihydroquinoline-3-carboxylic acid 30c was found to inhibit the Mtb isocitrate lyase (ICL) enzyme with in vitro MICs 0.16 and 0.04 μM against log- and starved-phase culture of Mtb and also showed good enzyme inhibition of Mtb ICL with IC50 of 0.10 ± 0.01 μM [167].
Cyclopropylphenyl derivatives
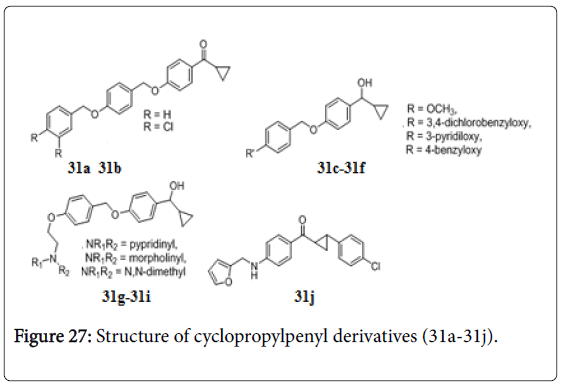
A series of cyclopropylphenylmethanone and cyclopropylphenylmethanol (31a-31j) (Figure 27), most of them possessed very good in vitro activity against both drug sensitive and drug resistant Mtb [168]. Compounds 31c, 31e, 31f, 31h and 31i have shown MIC 3.12 μg/mL, while compounds 31a, 31d and 34b exhibited MIC of 1.56, 1.56 and 0.78 μg/mL respectively. Compound 31g showed 98% killing of intracellular bacilli in mouse bone marrow derived macrophages and were active against MDR, XDR and rifampicin clinical isolates resistant strains with MIC 12.5 μg/mL. Compound 31g was orally active in vivo in mice against Mtb H37Rv [169]. A series of 4- alkylaminoaryl phenyl cyclopropyl methanones were also screened for their anti-TB activities against Mtb H37Rv. Compound 31j exhibited in vitro anti-TB activities with MIC values 3.12 μg/mL [170-175].
Conclusion
In recent years, the programs to control TB, extensive studies are made to enhance the anti-TB activity of new drugs particularly against resistant Mycobacterium strains. These advances in TB drug research and development are encouraging, but new drugs are needed that have strong, synergistic and complementary activities against various M. tuberculosis subpopulations in order to shorten TB treatment, be effective against MDRTB/XDR-TB, and be easily administered in conjunction with HIV. However, new targets should be further identified and discovered that can kill the viable Mtb in the latent phase and prevent the occurrence of resistance in bacterial cells.
References
- Asif M (2015) Anti-tubercular activity of some six membered heterocycle compounds. Chem Inter 1: 134-163.
- Asif M, Singh A, Lakshmayya (2013) The development of structurally different new antitubercular molecules containing pyridazine ring system. Chronicle Young Sci 4: 1-8.
- Asif M (2013) Some recent approaches in the design of synthetic anti-tubercular agents containing five membered heterocycles rings. Org Chem: An Indian J 9: 180-218.
- Asif M (2012) Recent efforts for the development of antitubercular drug containing diazine ring. Med Chem 2: 151-167.
- Asif M (2013) Development of new antitubercular drugs containing benz-fused ring system. Org Chem: An Indian J 9: 102-114.
- Asif M (2014) Antitubercular drugs: advances in nitrogen containing heterocyclic compounds and some other derivatives. J Pharm Chem 1: 37-43.
- Asif M (2012) Study of clinically used and recently developed antimycobacterial agents. Orien Pharm Experi Med 12: 15-34.
- Asif M (2012) Study of currently used antimycobacterials, their analogoues and recently developed agents. Indian drugs 49: 5-19.
- Asif M (2012) A review of antimycobacterial drugs in development. Mini Rev Med Chem 12: 1404-1418.
- Asif M (2012) A review on potent antitubercular agent isoniazid and its analogues. Inter J Pharm Chem 2: 110-120.
- Anand A, Upadhyaya K, Tripathi RP (2015) Drug development pipeline for the treatment of tuberculosis: Needs, challenges, success and opportunities for the future. Chem Biol Interface 5: 84-127.
- Surineni G, Yogeeswari P, Sriram D, Kantevari S (2016) Click-based synthesis and antitubercular evaluation of dibenzofuran tethered thiazolyl-1,2,3-triazolyl acetamides. Bioorg Med Chem Lett 26: 3684-3689.
- Sankar MM, Singh J, Diana SCA, Singh S (2013) Molecular characterization of Mycobacterium tuberculosis isolates from north Indian patients with extra pulmonary tuberculosis. Tuberculosis 93: 75-83.
- (2012) Global Tuberculosis Report 2012. World Health Organization, WHO Press, Geneva, Switzerland.
- Zhang Y, Post-Martens K, Denkin S (2006) New drug candidates and therapeutic targets for tuberculosis therapy. Drug Discov Today 11: 21-27.
- (2014) World Health Organization. Global tuberculosis report, 2014. Geneva, Switzerland.
- Zumla A, Raviglione M, Hafner R, von Reyn CF (2013) Tuberculosis. N Engl J Med 368: 745-755.
- (2014) Global tuberculosis report 2014. World Health Organization, Geneva.
- Falzon D, Gandhi N, Migliori GB, Sotgiu G, Cox HS, et al. (2013) Resistance to fluoroquinolones and second-line injectable drugs: impact on multidrug-resistant TB outcomes. Eur Respir J 42: 156-168.
- D’Ambrosio L, Centis R, Sotgiu G, Pontali E, Spanevello A, et al. (2015) New anti-tuberculosis drugs and regimens: 2015 update. ERJ Open Res 1: 00010-2015.
- Dhumal ST, Deshmukh AR, Bhosle MR, Khedkar VM, Nawale LU, et al. (2016) Synthesis and antitubercular activity of new 1,3,4-oxadiazoles bearing pyridyl and thiazolyl scaffolds. Bioorg Med Chem Lett 26: 3646-3651.
- Migliori GB, Sotgiu G (2014) Measuring the effect of tuberculosis control: a step forward. Lancet 383: 2026-2028.
- Tanimura T, Jaramillo E, Weil D, Raviglione M, Lönnroth K (2014) Financial burden for tuberculosis patients in low- and middle-income countries: a systematic review. Eur Respir J 43: 1763-1775.
- Weyer K, Mirzayev F, Migliori GB, Van Gemert W, D'Ambrosio L, et al. (2013) Rapid molecular TB diagnosis: evidence, policy making and global implementation of Xpert MTB/RIF. Eur Respir J 42: 252-271.
- Daskapan A, de Lange WC, Akkerman OW, Kosterink JG, van der Werf TS, et al. (2015) The role of therapeutic drug monitoring in individualised drug dosage and exposure measurement in tuberculosis and HIV co-infection. Eur Respir J 45: 569-571.
- Sassetti CM, Boyd DH, Rubin EJ (2003) Genes required for mycobacterial growth defined by high density mutagenesis. Mol Microbiol 48: 77-84.
- Joshi SM, Pandey AK, Capite N, Fortune SM, Rubin EJ, et al. (2006) Characterization of mycobacterial virulence genes through genetic interaction mapping. Proc Natl Acad Sci USA 103: 11760-11765.
- Mitchison DA (2000) Role of individual drugs in the chemotherapy of tuberculosis. Int J Tuberc Lung Dis 4: 796-806.
- Pham T, Nguyen T, Nguyen L (2013) Targeting Drug Resistance Mechanisms in Mycobacterium tuberculosis. J Anc Dis Prev Rem 1: 1-2.
- (2013) The use of bedaquiline in the treatment of multidrug-resistant tuberculosis, Interim Policy Guidance. WHO Press, World Health Organization: Geneva, Switzerland.
- Anishetty S, Pulimi M, Pennathur G (2005) Potential drug targets in Mycobacterium tuberculosis through metabolic pathway analysis. Comput Biol Chem 29: 368-378.
- (2010) The treatment of tuberculosis: guidelines - (4th edn.) Document WHO/HTM/TB/2009.420. Geneva, World Health Organization.
- Albanna AS, Smith BM, Cowan D, Menzies D (2013) Fixed-dose combination antituberculosis therapy: a systematic review and meta-analysis. Eur Respir J 42: 721-732.
- Erkin AV, Krutikov VI (2010) Effect of halogen atom localization on the level of antimycobacterial activity of 2-amino-4-arylamino-6-methylpyrimidines. Russ J Gen Chem 80: 818-824.
- Bacelar AH, Carvalho MA, Proença MF (2010) Synthesis and in vitro evaluation of substituted pyrimido[5,4-d]pyrimidines as a novel class of anti-Mycobacterium tuberculosis agents. Eur J Med Chem 45: 3234-3239.
- Geist JG, Lauw S, Illarionova V, Illarionov B, Fischer M, et al. (2010) Thiazolopyrimidine inhibitors of 2-methylerythritol 2,4-cyclodiphosphate synthase (IspF) from Mycobacterium tuberculosis and Plasmodium falciparum. Chem Med Chem 5: 1092-1101.
- Read ML, Braendvang M, Miranda PO, Gundersen LL (2010) Synthesis and biological evaluation of pyrimidine analogs of antimycobacterial purines. Bioorg Med Chem 18: 3885-3897.
- Anand N, Singh P, Sharma A, Tiwari S, Singh V, et al. (2012) Synthesis and evaluation of small libraries of triazolylmethoxy chalcones, flavanones and 2-aminopyrimidines as inhibitors of mycobacterial FAS-II and PknG. Bioorg Med Chem 20: 5150-5163.
- Yadlapalli RK, Chourasia OP, Vemuri K, Sritharan M, Perali RS (2012) Synthesis and in vitro anticancer and antitubercular activity of diarylpyrazole ligated dihydropyrimidines possessing lipophilic carbamoyl group. Bioorg Med Chem Lett 22: 2708-2711.
- Yokokawa F, Wang G, Chan WL, Ang SH, Wong J, et al. (2013) Discovery of tetrahydropyrazolopyrimidine carboxamide derivatives as potent and orally active antitubercular agents. ACS Med Chem Lett 4: 451-455.
- Shirude PS, Paul B, Roy Choudhury N, Kedari C, Bandodkar B, et al. (2012) Quinolinyl Pyrimidines: Potent Inhibitors of NDH-2 as a Novel Class of Anti-TB Agents. ACS Med Chem Lett 3: 736-740.
- Kumar RR, Perumal S, Senthilkumar P, Yogeeswari P, Sriram D (2008) Discovery of antimycobacterial spiro-piperidin-4-ones: an atom economic, stereoselective synthesis, and biological intervention. J Med Chem 51: 5731-5735.
- Karthikeyan SV, Bala BD, Raja VP, Perumal S, Yogeeswari P, et al. (2010) A highly atom economic, chemo-, regio- and stereoselective synthesis and evaluation of spiro-pyrrolothiazoles as antitubercular agents. Bioorg Med Chem Lett 20: 350-353.
- Das U, Das S, Bandy B, Stables JP, Dimmock JR (2008) N-Aroyl-3,5-bis(benzylidene)-4-piperidones: a novel class of antimycobacterial agents. Bioorg Med Chem 16: 3602-3607.
- Aridoss G, Amirthaganesan S, Kumar NA, Kim JT, Lim KT, et al. (2008) A facile synthesis, antibacterial, and antitubercular studies of some piperidin-4-one and tetrahydropyridine derivatives. Bioorg Med Chem Lett 18: 6542-6548.
- Carta A, Paglietti G, Rahbar Nikookar ME, Sanna P, Sechi L, et al. (2002) Novel substituted quinoxaline 1,4-dioxides with in vitro antimycobacterial and anticandida activity. Eur J Med Chem 37: 355-366.
- Ancizu S, Moreno E, Solano B, Villar R, Burguete A, et al. (2010) New 3-methylquinoxaline-2-carboxamide 1,4-di-N-oxide derivatives as anti-Mycobacterium tuberculosis agents. Bioorg Med Chem 18: 2713-2719.
- Das U, Das S, Bandy B, Gorecki DKJ, Dimmock JR (2010) E-2-[3-(3,4-Dichlorophenyl)-1-oxo-2-propenyl]-3-methylquinoxaline-1,4- dioxide: A lead antitubercular agent which alters mitochondrial respiration in rat liver. Eur J Med Chem 45: 4682-4686.
- Chopra S, Koolpe GA, Tambo-ong AA, Matsuyama KN, Ryan, KJ, et al. (2012) Discovery and optimization of benzotriazine di-n-oxides targeting replicating and nonreplicating Mycobacterium tuberculosis. J Med Chem 55: 6047-6060.
- Fassihi A, Azadpour Z, Delbari N, Saghaie L, Memarian HR, et al. (2009) Synthesis and antitubercular activity of novel 4-substituted imidazolyl-2,6-dimethyl-N3,N5-bisaryl-1,4-dihydropyridine-3,5-dicarboxamides. Eur J Med Chem 44: 3253-3258.
- Hulubei V, Meikrantz SB, Quincy DA, Houle T, McKenna JI, et al. (2012) 4-Isoxazolyl-1,4-dihydropyridines exhibit binding at the multidrug-resistance transporter. Bioorg Med Chem 20: 6613-6620.
- Manvar AT, Pissurlenkar RR, Virsodia VR, Upadhyay KD, Manvar DR, et al. (2010) Synthesis, in vitro antitubercular activity and 3D-QSAR study of 1,4-dihydropyridines. Mol Divers 14: 285-305.
- Trivedi AR, Dodiya DK, Dholariya BH, Kataria VB, Bhuva VR, et al. (2011) Synthesis and biological evaluation of some novel N-aryl-1,4-dihydropyridines as potential antitubercular agents. Bioorg Med Chem Lett 21: 5181-5183.
- Moraski GC, Markley LD, Chang M, Cho S, Franzblau SG, et al. (2012) Generation and exploration of new classes of antitubercular agents:The optimization of oxazolines, oxazoles, thiazolines, thiazoles to imidazo[1,2-a]pyridines and isomeric 5,6-fused scaffolds. Bioorg Med Chem 20: 2214-2220.
- Ramachandran S, Panda M, Mukherjee, K, Choudhury NR, Tantry SJ, et al. (2013) Synthesis and structure activity relationship of imidazo[1,2-a] pyridine-8-carboxamides as a novel antimycobacterial lead series. Bioorg Med Chem Lett 23: 4996-5001.
- Moraski GC, Markley LD, Hipskind PA, Boshoff H, Cho S, et al. (2011) Advent of Imidazo[1,2-a]pyridine-3-carboxamides with Potent Multi- and Extended Drug Resistant Antituberculosis Activity. ACS Med Chem Lett 2: 466-470.
- Moraski GC, Markley LD, Cramer J, Hipskind PA, Boshoff H, et al. (2013) Advancement of imidazo[1,2-a]pyridines with improved pharmacokinetics and nM activity vs. Mycobacterium tuberculosis. ACS Med Chem Lett 4: 675-679.
- Samala G, Devi PB, Nallangi R, Yogeeswari P, Sriram D (2013) Development of 3-phenyl-4,5,6,7-tetrahydro-1H-pyrazolo[4,3-c]pyridine derivatives as novel Mycobacterium tuberculosis pantothenate synthetase inhibitors. Eur J Med Chem 69: 356-364.
- Tripathi RP, Tripathi R, Tiwari VK, Bala L, Sinha S, et al. (2002) Synthesis of glycosylated beta-amino acids as new class of antitubercular agents. Eur J Med Chem 37: 773-781.
- Alvey L, Prado S, Huteau V, Saint-Joanis B, Michel S, et al. (2008) A new synthetic access to furo[3,2-f]chromene analogues of an antimycobacterial. Bioorg Med Chem 16: 8264-8272.
- Alvey L, Prado S, Saint-Joanis B, Michel S, Koch M, et al. (2009) Diversity-oriented synthesis of furo[3,2-f]chromanes with antimycobacterial activity. Eur J Med Chem 44: 2497-2505.
- Kumar RR, Perumal S, Menéndez JC, Yogeeswari P, Sriram D (2011) Anti-mycobacterial activity of novel 1,2,4-oxadiazole pyranopyridine/chromene hybrids generated by chemoselective 1,3-dipolar cycloadditions of nitrile oxides. Bioorg Med Chem 19: 3444-3450.
- Sriram D, Yogeeswari P, Dinakaran M, Banerjee D, Bhat P, et al. (2010) Discovery of novel antitubercular 2,10-dihydro-4aH-chromeno[3,2-c]pyridin-3-yl derivatives. Eur J Med Chem 45: 120-123.
- Jeyachandran M, Ramesh P, Sriram D, Senthilkumar P, Yogeeswari P (2012) Synthesis and in vitro antitubercular activity of 4 -aryl/alkylsulfonyl methylcoumarins as inhibitors of Mycobacterium tuberculosis. Bioorg Med Chem Lett 22: 4807-4809.
- Kawate T, Iwase N, Shimizu M, Stanley SA, Wellington S, et al. (2013) Synthesis and structure-activity relationships of phenyl-substituted coumarins with anti-tubercular activity that target FadD32. Bioorg Med Chem Lett 23: 6052-6059.
- Bonde CG, Gaikwad NJ (2004) Synthesis and preliminary evaluation of some pyrazine containing thiazolines and thiazolidinones as antimicrobial agents. Bioorg Med Chem 12: 2151-2161.
- Huang Q, Mao J, Wan B, Wang Y, Brun R, et al. (2009) Searching for new cures for tuberculosis: design, synthesis, and biological evaluation of 2-methylbenzothiazoles. J Med Chem 52: 6757-6767.
- Turan-Zitouni G, Kaplancikli ZA, Ozdemir A (2010) Synthesis and antituberculosis activity of some N-pyridyl-N'-thiazolylhydrazine derivatives. Eur J Med Chem 45: 2085-2088.
- Suresh Kumar GV, Rajendraprasad Y, Mallikarjuna BP, Chandrashekar SM, Kistayya C (2010) Synthesis of some novel 2-substituted-5-[isopropylthiazole] clubbed 1,2,4-triazole and 1,3,4-oxadiazoles as potential antimicrobial and antitubercular agents. Eur J Med Chem 45: 2063-2074.
- Güzeldemirci NU, Küçükbasmaci O (2010) Synthesis and antimicrobial activity evaluation of new 1,2,4-triazoles and 1,3,4-thiadiazoles bearing imidazo[2,1-b]thiazole moiety. Eur J Med Chem 45: 63-68.
- Karoli T, Becker B, Zuegg J, Möllmann U, Ramu S, et al. (2012) Identification of antitubercular benzothiazinone compounds by ligand-based design. J Med Chem 55: 7940-7944.
- Meissner A, Boshoff HI, Vasan M, Duckworth BP, Barry CE 3rd, et al. (2013) Structure-activity relationships of 2-aminothiazoles effective against Mycobacterium tuberculosis. Bioorg Med Chem 21: 6385-6397.
- Gao C, Ye T-H, Wang N-Yu, Zeng X-X, Zhang Li-D, et al. (2013) Synthesis and structure-activity relationships evaluation of benzothiazinone derivatives as potential anti-tubercular agents. Bioorg Med Chem Lett 23: 4919-4922.
- Yang J, Pi W, Xiong L, Ang W, Yang T, et al. (2013) 3H-1,2,4-Dithiazol-3-one compounds as novel potential affordable antitubercular agents. Bioorg Med Chem Lett 23: 1424-1427.
- Deidda D, Lampis G, Fioravanti R, Biava M, Porretta GC, et al. (1998) Bactericidal activities of the pyrrole derivative BM212 against multidrug-resistant and intramacrophagic Mycobacterium tuberculosis strains. Antimicrob Agents Chemother 42: 3035-3037.
- Biava M, Porretta GC, Poce G, De Logu A, Meleddu R, et al. (2009) 1,5-Diaryl-2-ethyl pyrrole derivatives as antimycobacterial agents: design, synthesis, and microbiological evaluation. Eur J Med Chem 44: 4734-4738.
- Joshi SD, Vagdevi HM, Vaidya VP, Gadaginamath GS (2008) Synthesis of new 4-pyrrol-1-yl benzoic acid hydrazide analogs and some derived oxadiazole, triazole and pyrrole ring systems: a novel class of potential antibacterial and antitubercular agents. Eur J Med Chem 43: 1989-1996.
- Hearn MJ, Chen MF, Terrot MS, Webster ER, Cynamon MH (2010) Preparation and properties in vitro and in vivo of antitubercular pyrroles. J Het Chem 47: 707-712.
- Almansour AI, Ali S, Ali MA, Ismail R, Choon TS, et al. (2012) A regio- and stereoselective 1,3-dipolar cycloaddition for the synthesis of new-fangled dispiropyrrolothiazoles as antimycobacterial agents. Bioorg Med Chem Lett 22: 7418-7421.
- Moraski GC, Franzblau SG, Miller MJ (2010) Utiliztion of the suzuki coupling to enhance the antituberculosis activity of aryl oxazoles. Heterocycles 80: 977-988.
- Rane RA, Gutte SD, Sahu NU (2012) Synthesis and evaluation of novel 1,3,4-oxadiazole derivatives of marine bromopyrrole alkaloids as antimicrobial agent. Bioorg Med Chem Lett 22: 6429-6432.
- Almansour AI, Kumar RS, Arumugam N, Sriram D (2012) A solvent free, four-component synthesis and 1,3-dipolar cycloaddition of 4(H)-pyrans with nitrile oxides: Synthesis and discovery of antimycobacterial activity of enantiomerically pure 1,2,4-oxadiazoles. Eur J Med Chem 53: 416-423.
- Moura KCG, Carneiro PF, Pinto M do CFR, da Silva JA, Malta VRS, et al. (2012) 1,3-Azoles from ortho-naphthoquinones: Synthesis of aryl substituted imidazoles and oxazoles and their potent activity against Mycobacterium tuberculosis. Bioorg Med Chem 20: 6482-6488.
- Suresh Kumar GV, Rajendraprasad Y, Mallikarjuna BP, Chandrashekar SM, Kistayya C (2010) Synthesis of some novel 2-substituted-5-[isopropylthiazole] clubbed 1,2,4-triazole and 1,3,4-oxadiazoles as potential antimicrobial and antitubercular agents. Eur J Med Chem 45: 2063-2074.
- Bakal RL, Gattani SG (2012) Identification and development of 2,5-disubstituted oxadiazole as potential candidate for treatment of XDR and MDR tuberculosis. Eur J Med Chem 47: 278-282.
- Flipo M, Desroses M, Lecat-Guillet N, Carette BDX, Leroux F, et al. (2011) Ethionamide boosters: synthesis, biological activity, and structure activity relationships of a series of 1,2,4-oxadiazole EthR inhibitors. J Med Chem 54: 2994-3010.
- Tangallapally RP, Sun D, Rakesh, Budha N, Lee RE, et al. (2007) Discovery of novel isoxazolines as anti-tuberculosis agents. Bioorg Med Chem Lett 17: 6638-6642.
- Barbachyn MR, Cleek GJ, Dolak LA, Garmon SA, Morris J, et al. (2003) Identification of phenylisoxazolines as novel and viable antibacterial agents active against Gram-positive pathogens. J Med Chem 46: 284-302.
- Tripathi RP, Yadav AK, Ajay A, Bisht SS, Chaturvedi V, et al. (2010) Application of Huisgen (3+2) cycloaddition reaction: synthesis of 1-(2,3-dihydrobenzofuran-2-yl-methyl [1,2,3]-triazoles and their antitubercular evaluations. Eur J Med Chem 45: 142-148.
- Singh BK, Yadav AK, Kumar B, Gaikwad A, Sinha SK, et al. (2008) Preparation and reactions of sugar azides with alkynes: synthesis of sugar triazoles as antitubercular agents. Carbohydr Res 343: 1153-1162.
- Costa MS, Boechat N, Rangel EA, da Silva Fde C, de Souza AM, et al. (2006) Synthesis, tuberculosis inhibitory activity, and SAR study of N-substituted-phenyl-1,2,3-triazole derivatives. Bioorg Med Chem 14: 8644-8653.
- Menendez C, Chollet A, Rodriguez F, Inard C, Pasca MR, et al. (2012) Chemical synthesis and biological evaluation of triazole derivatives as inhibitors of InhA and antituberculosis agents. Eur J Med Chem 52: 275-283.
- Mugunthan G, Ramakrishna K, Sriram D, Yogeeswari, Kartha KPR (2011) Synthesis and screening of galactose-linked nitroimidazoles and triazoles against Mycobacterium tuberculosis. Eur J Med Chem 46: 4725-4732.
- Carta A, Palomba M, Briguglio I, Corona P, Piras S, et al. (2011) Synthesis and anti-mycobacterial activities of triazoloquinolones. Eur J Med Chem 46: 320-326.
- Thomas KD, Adhikari AV, Chowdhury IH, Sumesh E, Pal NK (2011) New quinolin-4-yl-1,2,3-triazoles carrying amides, sulphonamides and amidopiperazines as potential antitubercular agents. Eur J Med Chem 46: 2503-2512.
- Patpi SR, Pulipati L, Yogeeswari P, Sriram D, Jain N, et al. (2012) Design, synthesis, and structure-activity correlations of novel dibenzo[b,d]furan, dibenzo[b,d]thiophene, and N-methylcarbazole clubbed 1,2,3-triazoles as potent inhibitors of Mycobacterium tuberculosis. J Med Chem 55: 3911-3922.
- Menendez C, Rodriguez F, Ribeiro AL, Zara F, Frongia C, et al. (2013) Synthesis and evaluation of α-ketotriazoles and α,β-diketotriazoles as inhibitors of Mycobacterium tuberculosis. Eur J Med Chem 69: 167-173.
- Kim P, Zhang L, Manjunatha UH, Singh R, Patel S, et al. (2009) Structure-activity relationships of anti-tubercular nitroimidazoles. 1 structural features associated with aerobic and anaerobic activities of 4- and 5-nitroimidazoles. J Med Chem 52: 1317-1328.
- Lee SH, Kim S, Yun MH, Lee YS, Cho SN, et al. (2011) Synthesis and antitubercular activity of monocyclic nitroimidazoles: insights from econazole. Bioorg Med Chem Lett 21: 1515-1518.
- Alegaon SG, Alagawadi KR, Sonkusare PV, Chaudhary SM, Dadwe DH, et al. (2012) Novel imidazo[2,1-b][1,3,4]thiadiazole carrying rhodanine-3-acetic acid as potential antitubercular agents. Bioorg Med Chem Lett 22: 1917-1921.
- Sharma GK, Pathak D (2010) 3D-QSAR study on ring substituted imidazoles for their antitubercular activity. Drug Design Discovery 7: 128-132.
- Sivendran S, Jones V, Sun D, Wang Y, Grzegorzewicz AE, et al. (2010) Identification of triazinoindol-benzimidazolones as nanomolar inhibitors of the Mycobacterium tuberculosis enzyme TDP-6-deoxy-d-xylo-4-hexopyranosid-4-ulose 3,5-epimerase (RmlC). Bioorg Med Chem 18: 896-908.
- Pandey J, Tiwari VK, Verma SS, Chaturvedi V, Bhatnagar S, et al. (2009) Synthesis and antitubercular screening of imidazole derivatives. Eur J Med Chem 44: 3350-3355.
- Tukulula M, Sharma RK, Meurillon M, Mahajan A, Naran K, et al. (2012) Synthesis and antiplasmodial and antimycobacterial evaluation of new nitroimidazole and nitroimidazooxazine derivatives. ACS Med Chem Lett 4: 128-131.
- Pathak RB, Chovatia PT, Parekh HH (2012) Synthesis, antitubercular and antimicrobial evaluation of 3-(4-chlorophenyl)-4-substituted pyrazole derivatives. Bioorg Med Chem Lett 22: 5129-5133.
- Ahsan MJ, Samy JG, Jain CB, Dutt KR, Khalilullah H, et al. (2012) Discovery of novel antitubercular 1,5-dimethyl-2-phenyl-4-([5-(arylamino)-1,3,4-oxadiazol-2-yl]methylamino)-1,2-dihydro-3H-pyrazol-3-one analogues. Bioorg Med Chem Lett 22: 969-972.
- Gunasekaran P, Perumal S, Yogeeswari P, Sriram D (2011) A facile four-component sequential protocol in the expedient synthesis of novel 2-aryl-5-methyl-2,3-dihydro-1H-3-pyrazolones in water and their antitubercular evaluation. Eur J Med Chem 46: 4530-4536.
- Palmer BD, Thompson AM, Sutherland HS, Blaser A, Kmentova I, et al. (2010) Synthesis and structure-activity studies of biphenyl analogues of the tuberculosis drug (6S)- 2-nitro-6-{[4-(trifluoromethoxy)benzyl]oxy}-6,7-dihydro-5Himidazo[2,1-b][1,3]oxazine (PA-824). J Med Chem 53: 282-294.
- Kmentova I, Sutherland HS, Palmer BD, Blaser A, Franzblau SG, et al. (2010) Synthesis and structure-activity relationships of aza- and diazabiphenyl analogues of the antitubercular drug (6S)-2-nitro-6-{[4-(trifluoromethoxy)benzyl]oxy}-6,7-dihydro-5H-imidazo[2,1b][1,3]oxazine (PA-824). J Med Chem 53: 8421-8439.
- Cherian J, Choi I, Nayyar A, Manjunatha UH, Mukherjee T, et al. (2011) Structure-activity relationships of antitubercular nitroimidazoles. 3. Exploration of the linker and lipophilic tail of ((s)-2-nitro-6,7-dihydro-5H-imidazo[2,1-b][1,3]oxazin-6-yl)-(4-trifluoromethoxybenzyl)amine (6-amino PA-824). J Med Chem 54: 5639-5659.
- Blaser A, Palmer BD, Sutherland HS, Kmentova I, Franzblau SG, et al. (2012) Structure-activity relationships for amide-, carbamate-, and urea-linked analogues of the tuberculosis drug (6S)-2-nitro-6-{[4-(trifluoromethoxy)benzyl]oxy}-6,7-dihydro-5H-imidazo[2,1-b][1,3]oxazine (PA-824). J Med Chem 55: 312-326.
- Thompson AM, Sutherland HS, Palmer BD, Kmentova I, Blaser A, et al. (2011) Synthesis and structure-activity relationships of varied ether linker analogues of the antitubercular drug (6S)-2-nitro-6-{[4-(trifluoromethoxy)benzyl]oxy}-6,7-dihydro-5h-imidazo[2,1-b][1,3]oxazine (PA-824). J Med Chem 54: 6563-6585.
- Sutherland HS, Blaser A, Kmentova I, Franzblau SG, Wan B, et al. (2010) Synthesis and structure-activity relationships of antitubercular 2-nitroimidazooxazines bearing heterocyclic side chains. J Med Chem 53: 855-866.
- Anand N, Ramakrishna KK, Gupt MP, Chaturvedi V, Singh S, et al. (2013) Identification of 1-[4-Benzyloxyphenyl)-but-3-enyl]-1H-azoles as New Class of Antitubercular and Antimicrobial Agents. ACS Med Chem Lett 4: 958-963.
- de Souza MV, Pais KC, Kaiser CR, Peralta MA, de L Ferreira M, et al. (2009) Synthesis and in vitro antitubercular activity of a series of quinoline derivatives. Bioorg Med Chem 17: 1474-1480.
- Upadhayaya RS, Kulkarni GM, Vasireddy NR (2009) Design synthesis and biological evaluation of novel triazole, urea and thiourea derivatives of quinoline against Mycobaterium tuberculosis. Bioorg Med Chem 17: 4681-4692.
- Gemma S, Savini L, Altarelli M, Tripaldi P, Chiasserini L, et al. (2009) Development of antitubercular compounds based on a 4-quinolylhydrazone scaffold. Further structure-activity relationship studies. Bioorg Med Chem 17: 6063-6072.
- Eswaran S, Adhikari AV, Pal NK, Chowdhury IH (2010) Design and synthesis of some new quinoline-3-carbohydrazone derivatives as potential antimycobacterial agents. Bioorg Med Chem Lett 20: 1040-1044.
- Balamurugan K, Jeyachandran V, Perumal S, Manjashetty TH, Yogeeswari P (2010) A microwave-assisted, facile, regioselective Friedlander synthesis and antitubercular evaluation of 2,9-diaryl-2,3-dihydrothieno-[3,2-b]quinolines. Eur J Med Chem 45: 682-688.
- Upadhayaya RS, Vandavasi JK, Kardile RA, Lahore SV, Dixit SS, et al. (2010) Novel quinoline and naphthalene derivatives as potent antimycobacterial agents. Eur J Med Chem 45: 1854-1867.
- Shah NM, Patel MP, Patel RG (2012) New N-arylamino biquinoline derivatives: synthesis, antimicrobial, antituberculosis, and antimalarial evaluation. Eur J Med Chem 54: 239-247.
- Jain PP, Degani MS, Raju A, Ray M, Rajan MG (2013) Rational drug design based synthesis of novel arylquinolines as anti-tuberculosis agents. Bioorg Med Chem Lett 23: 6097-6105.
- Mulchin BJ, Newton CG, Baty JW, Grasso CH, Martin WJ, et al. (2010) The anti-cancer, anti-inflammatory and tuberculostatic activities of a series of 6,7-substituted-5,8-quinolinequinones. Bioorg Med Chem 18: 3238-3251.
- Upadhayaya RS, Lahore SV, Sayyed AY, Dixit SS, Shind PD (2010) Conformationally-constrained indeno[2,1-c]quinolines a new class of anti-mycobacterial agents. Org Biomol Chem 8: 2180-2197.
- Eswaran S, Adhikari AV, Ajay Kumar R (2010) New 1,3-oxazolo[4,5-c]quinoline derivatives: synthesis and evaluation of antibacterial and antituberculosis properties. Eur J Med Chem 45: 957-966.
- Mao J, Yuan H, Wang Y, Wan B, Pak D, et al. (2010) Synthesis and antituberculosis activity of novel mefloquine-isoxazole carboxylic esters as prodrugs. Bioorg Med Chem Lett 20: 1263-1268.
- Eswaran S, Adhikari AV, Chowdhury IH, Pal NK, Thomas KD (2010) New quinoline derivatives: synthesis and investigation of antibacterial and antituberculosis properties. Eur J Med Chem 45: 3374-3383.
- Eswaran S, Adhikari AV, Pal NK, Chowdhury IH (2010) Design and synthesis of some new quinoline-3-carbohydrazone derivatives as potential antimycobacterial agents. Bioorg Med Chem Lett 20: 1040-1044.
- Yang CL, Tseng CH, Chen YL, Lu CM, Kao CL, et al. (2010) Identification of benzofuro[2,3-b]quinoline derivatives as a new class of antituberculosis agents. Eur J Med Chem 45: 602-607.
- Thomas KD, Adhikari AV, Chowdhury IH, Sandeep T, Mahmood R, et al. (2011) Design, synthesis and docking studies of quinoline-oxazolidinone hybrid molecules and their antitubercular properties. Eur J Med Chem 46: 4834-4845.
- Paul N, Murugavel M, Muthusubramanian S, Sriram D (2012) Camphorsulfonic acid catalysed facile tandem double Friedlander annulation protocol for the synthesis of phenoxy linked bisquinoline derivatives and discovery of antitubercular agents. Bioorg Med Chem Lett 22: 1643-1648.
- Prasanna P, Balamurugan K, Perumal S, Yogeeswari P, Sriram D (2010) A regio- and stereoselective 1,3-dipolar cycloaddition for the synthesis of novel spiro-pyrrolothiazolyloxindoles and their antitubercular evaluation. Eur J Med Chem 45: 5653-5661.
- Onajole OK, Pieroni M, Tipparaju SK, Lun S, Stec J, et al. (2013) Preliminary structure-activity relationships and biological evaluation of novel antitubercular indolecarboxamide derivatives against drug-susceptible and drug-resistant Mycobacterium tuberculosis strains. J Med Chem 56: 4093-4103.
- Aboul-Fadl T, Bin-Jubair FA, Aboul-Wafa O (2010) Schiff bases of indoline-2,3-dione (isatin) derivatives and nalidixic acid carbohydrazide, synthesis, antitubercular activity and pharmacophoric model building. Eur J Med Chem 45: 4578-4586.
- Kumar S, Dwivedi AP, Kashyap VK, Saxena AK, Dwivedi AK, et al. (2013) Synthesis and biological evaluation of trans 6-methoxy-1,1-dimethyl-2-phenyl-3-aryl-2,3-dihydro-1H-inden-4-yloxyalkylamine derivatives against drug susceptible, non-replicating M. tuberculosis H37Rv and clinical multidrug resistant strains. Bioorg Med Chem Lett 23: 2404-2407.
- Kumar K, Awasthi D, Lee SY, Zanardi I, Ruzsicska B, et al. (2011) Novel trisubstituted benzimidazoles, targeting Mtb FtsZ, as a new class of antitubercular agents. J Med Chem 54: 374-381.
- Gobis K, Foks H, Bojanowski K, Augustynowicz-Kopec E, Napiorkowska A (2012) Synthesis of novel 3-cyclohexylpropanoic acid-derived nitrogen heterocyclic compounds and their evaluation for tuberculostatic activity. Bioorg Med Chem 20: 137-144.
- Tawari NR, Bairwa R, Ray MK, Rajan MG, Degani MS (2010) Design, synthesis, and biological evaluation of 4-(5-nitrofuran-2-yl)prop-2-en-1-one derivatives as potent antitubercular agents. Bioorg Med Chem Lett 20: 6175-6178.
- Kantevari S, Yempala T, Yogeeswari P, Sriram D, Sridhar B (2011) Synthesis and antitubercular evaluation of amidoalkyl dibenzofuranols and 1H-benzo[2,3]benzofuro[4,5-e][1,3]oxazin-3(2H)-ones. Bioorg Med Chem Lett 21: 4316-4319.
- De P, Koumba Yoya G, Constant P, Bedos-Belval F, Duran H, et al. (2011) Design, synthesis, and biological evaluation of new cinnamic derivatives as antituberculosis agents. J Med Chem 54: 1449-1461.
- Limaye RA, Kumbhar VB, Natu AD, Paradkar MV, Honmore VS, et al. (2013) One pot solvent free synthesis and in vitro antitubercular screening of 3-Aracylphthalides against Mycobacterium tuberculosis. Bioorg Med Chem Lett 23: 711-714.
- Potewar TM, Ingale SA, Srinivasan KV (2008) Synthesis of tryptanthrin and deoxyvasicinone by a regioselective lithiation-intramolecular electophillic reaction approach. Arkivoc 14: 100-108.
- Liu YX, Xiao CL, Wang YX, Li YH, Yang YH, et al. (2012) Synthesis, structure-activity relationship and in vitro anti-mycobacterial evaluation of 13-n-octylberberine derivatives. Eur J Med Chem 52: 151-158.
- Tripathi RP, Verma SS, Pandey J, Agarwal KC, Chaturvedi V, et al. (2006) Search of antitubercular activities in tetrahydroacridines: synthesis and biological evaluation. Bioorg Med Chem Lett 16: 5144-5147.
- Katiyar D, Tiwari VK, Tewari N, Verma SS, Sinha S, et al. (2005) Synthesis and antimycobacterial activities of glycosylated amino alcohols and amines. Eur J Med Chem 40: 351-360.
- Tripathi RP, Tiwari VK, Tewari N, Katiyar D, Saxena N, et al. (2005) Synthesis and antitubercular activities of bis-glycosylated diamino alcohols. Bioorg Med Chem 13: 5668-5679.
- Tripathi RP, Saxena N, Tiwari VK, Verma SS, Chaturvedi V, et al. (2006) Synthesis and antitubercular activity of substituted phenylmethyl- and pyridylmethyl amines. Bioorg Med Chem 14: 8186-8196.
- Singh N, Pandey J, Yadav A, Chaturvedi V, Bhatnagar S, et al. (2009) A facile synthesis of a,a’(EE)-bis(benzylidene)-cycloalkanones and their antitubercular evaluations. Eur J Med Chem 44: 1705-1709.
- Katiyar D, Tiwari VK, Tripathi RP, Srivastava A, Chaturvedi V, et al. (2003) Synthesis and antimycobacterial activity of 3,5-disubstituted thiadiazine thiones. Bioorg Med Chem 11: 4369-4375.
- Pathak AK, Pathak V, Seitz LE, Suling WJ, Reynolds RC (2013) 6-Oxo and 6-thio purine analogs as antimycobacterial agents. Bioorg Med Chem 21: 1685-1695.
- Li X, Liu N, Zhang H, Knudson SE, Li H-J, et al. (2011) CoA Adducts of 4-Oxo-4-phenylbut-2-enoates: Inhibitors of MenB from the M. tuberculosis Menaquinone biosynthesis pathway. ACS Med Chem Lett 2: 818-823.
- Matyugina E, Khandazhinskaya A, Chernousova L, Andreevskaya S, Smirnova T, et al. (2012) The synthesis and antituberculosis activity of 5'-nor carbocyclic uracil derivatives. Bioorg Med Chem 20: 6680-6686.
- Phetsuksiri B, Baulard AR, Cooper AM, Minnikin DE, Douglas JD, et al. (1999) Antimycobacterial activities of isoxyl and new derivatives through the inhibition of mycolic acid synthesis. Antimicrob Agents Chemother 43: 1042-1051.
- Gannoun-Zaki L, Alibaud L, Kremer L (2013) Point mutations within the fatty acid synthase type II dehydratase components HadA or HadC contribute to isoxyl resistance in Mycobacterium tuberculosis. Antimicrob Agents Chemother 57: 629-632.
- Brown JR, North EJ, Hurdle JG, Morisseau C, Scarborough JS, et al. (2011) The structure-activity relationship of urea derivatives as anti-tuberculosis agents. Bioorg Med Chem 19: 5585-5595.
- North EJ, Scherman MS, Bruhn DF, Scarborough JS, Maddox MM, et al. (2013) Design, synthesis and anti-tuberculosis activity of 1-adamantyl-3-heteroaryl ureas with improved in vitro pharmacokinetic properties. Bioorg Med Chem 21: 2587-2599.
- Singh S, Mandal PK, Singh N (2010) Substituted hydrazine carbothioamide as potent antitubercular agents: Synthesis and quantitative structure-activity relationship (QSAR). Bioorg Med Chem Lett 20: 2597-2600.
- Vavríková E, Polanc S, Kocevar M, Horváti K, Bosze S, et al. (2011) New fluorine-containing hydrazones active against MDR-tuberculosis. Eur J Med Chem 46: 4937-4945.
- Silveira GP, Ferreira M, Fernandes L, Moraski GC, Cho S, et al. (2012) Allylic thiocyanates as a new class of antitubercular agents. Bioorg Med Chem Lett 22: 6486-6489.
- Koçyigit KB, Rollas S (2002) Synthesis, characterization and evaluation of antituberculosis activity of some hydrazones. Farmaco 57: 595-599.
- Savini L, Chiasserini L, Gaeta A, Pellerano C (2002) Synthesis and anti-tubercular evaluation of 4-quinolylhydrazones. Bioorg Med Chem 10: 2193-2198.
- Sonar VN, Crooks PA (2009) Synthesis and antitubercular activity of a series of hydrazone and nitrovinyl analogs derived from heterocyclic aldehydes. J Enzyme Inhib Med Chem 24: 117-124.
- Logua AD, Saddi M, Onnis V (2005) In vitro antimycobacterial activity of newly synthesized S-alkylisothiosemicarbazone derivatives and synergistic interactions in combination with rifamycins against Mycobacterium avium. Int J Antimicrob Agents 26: 28-32.
- Ballell L, Field RA, Duncan K, Young RJ (2005) New small-molecule synthetic antimycobacterials. Antimicrob Agents Chemother 49: 2153-2163.
- D'Oca Cda R, Coelho T, Marinho TG, Hack CR, Duarte Rda C, et al. (2010) Synthesis and antituberculosis activity of new fatty acid amides. Bioorg Med Chem Lett 20: 5255-5257.
- Sriram D, Yogeeswari P, Methuku S, Vyas DR, Senthilkumar P, et al. (2011) Synthesis of various 3-nitropropionamides as Mycobacterium tuberculosis isocitrate lyase inhibitor. Bioorg Med Chem Lett 21: 5149-5154.
- Dwivedi N, Tewari N, Tiwari VK, Chaturvedi V, Manju YK, et al. (2005) An efficient synthesis of aryloxyphenyl cyclopropyl methanones: a new class of anti-mycobacterial agents. Bioorg Med Chem Lett 15: 4526-4530.
- Bisht SS, Dwivedi N, Chaturvedi V, Anand N, Misra M, et al. (2010) Synthesis and optimization of antitubercular activities in a series of 4-(aryloxy) phenyl cyclopropyl methanols. Eur J Med Chem 45: 5965-5978.
- Ajay A, Singh V, Singh S, Pandey S, Gunjan S, et al. (2010) Synthesis and bio-evaluation of alkylaminoaryl phenyl cyclopropyl methanones as antitubercular and antimalarial agents. Bioorg Med Chem 18: 8289-8301.
- De Logu A, Saddi M, Onnis V, Sanna C, Congiu C, et al. (2005) In vitro antimycobacterial activity of newly synthesised S-alkylisothiosemicarbazone derivatives and synergistic interactions in combination with rifamycins against Mycobacterium avium. Int J Antimicrob Agents 26: 28-32.
- D'Oca Cda R, Coelho T, Marinho TG, Hack CR, Duarte Rda C, et al. (2010) Synthesis and antituberculosis activity of new fatty acid amides. Bioorg Med Chem Lett 20: 5255-5257.
- Sriram D, Yogeeswari P, Methuku S, Vyas DR, Senthilkumar P, et al. (2011) Synthesis of various 3-nitropropionamides as Mycobacterium tuberculosis isocitrate lyase inhibitor. Bioorg Med Chem Lett 21: 5149-5154.
- Dwivedi N, Tewari N, Tiwari VK, Chaturvedi V, Manju YK, et al. (2005) An efficient synthesis of aryloxyphenyl cyclopropyl methanones: a new class of anti-mycobacterial agents. Bioorg Med Chem Lett 15: 4526-4530.
- Bisht SS, Dwivedi N, Chaturvedi V, Anand N, Misra M, et al. (2010) Synthesis and optimization of antitubercular activities in a series of 4-(aryloxy) phenyl cyclopropyl methanols. Eur J Med Chem 45: 5965-5978.
- Ajay A, Singh V, Singh S, Pandey S, Gunjan S, et al. (2010) Synthesis and bio-evaluation of alkylaminoaryl phenyl cyclopropyl methanones as antitubercular and antimalarial agents. Bioorg Med Chem 18: 8289-8301.
Copyright: © 2016 Asif M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.