Journal of Cancer Research and Immuno-Oncology
Open Access
ISSN: 2684-1266
ISSN: 2684-1266
Case Report - (2021)Volume 7, Issue 1
Objective: The aim of this study is to highlight the hospital course of a pediatric patient with concurrent sickle cell trait, alpha thalassemia, and G6PD deficiency.
Methods: The patient’s direct bilirubin remained less than 0.2 mg/dl throughout his hospitalization but his total bilirubin peaked at 18.7 mg/dl at 84 hours of life. While the patient’s bilirubin levels decreased after this, the decline was not as rapid as anticipated so a peripheral smear was performed which showed spherocytosis. Hemoglobin electrophoresis was also conducted just prior to discharge after the patient’s hyperbilirubinemia had resolved.
Results: The results of the patient’s hemoglobin electrophoresis revealed that the patient was a sickle cell trait carrier and also showed evidence of trace Hb Barts consistent with alpha thalassemia. In addition, given the patient’s peripheral smear showing spherocytosis, G6PD levels were also assessed and found to be low consistent with mild to moderate G6PD deficiency. The patient’s family was educated about precautions to take to reduce the risk of excessive oxidative stress that could precipitate acute hemolytic anemia episodes in the future.
Conclusion: The concurrent presentation of sickle cell trait, alpha thalassemia, and G6PD deficiency is rare and it is theorized that each trait respectively confers an evolutionary advantage against malaria.
Sickle cell trait; Alpha thalassemia; G6PD deficiency; Hemoglobin electrophoresis
Sickle Cell Trait (SCT) is an inherited blood disorder. Alpha thalassemia is a blood disorder that reduces the production of haemoglobin. G6PD deficiency is a genetic disorder that most often affects males. It happens when the body doesn't have enough of an enzyme called glucose-6-phosphate dehydrogenase (G6PD). G6PD helps red blood cells work. It also protects them from substances in the blood that could harm them.
An estimated 3 million people in the U.S. have sickle cell trait but the exact prevalences of alpha thalassemia and glucose-6-phosphate dehydrogenase (G6PD) deficiency in the U.S. are not well known [1]. An estimated 10% of black males in the U.S. are thought to have G6PD deficiency which is an X-linked recessive disease more common in Africa, Asia, and the Middle East [2]. An estimated 5% of the world’s population is thought to carry a variant of the alpha thalassemia trait and prevalence in the U.S. is thought to be increasing in recent years [3]. While reports of sickle cell trait co-presenting with either alpha thalassemia or G6PD deficiency have been noted in previous studies, documented cases of all three inherited blood disorders presenting together are exceedingly rare.
A 4 months old boy born full-term was referred to the hematology clinic following persistent neonatal hyperbilirubinemia during the first week of life. His mother is a sickle cell trait carrier but had an otherwise unremarkable pregnancy course and received routine prenatal care. The baby was first admitted to the hospital after being found to have a total bilirubin level of 16.8 mg/dl at 76 hours of life. Because the infant had been breastfeeding and stooling appropriately for his age, he was initially thought to have either breast feeding or breast milk jaundice and admitted for phototherapy.
The patient’s direct bilirubin remained less than 0.2 mg/dl throughout his hospitalization but his total bilirubin peaked at 18.7 mg/dl at 84 hours of life. While the patient’s bilirubin levels decreased after this, the decline was not as rapid as anticipated so a peripheral smear was performed which showed spherocytosis. Haemoglobin electrophoresis was also conducted just prior to discharge after the patient’s hyperbilirubinemia had resolved.
The results of the patient’s haemoglobin electrophoresis revealed that the patient was a sickle cell trait carrier and also showed evidence of trace Hb Barts consistent with alpha thalassemia. In addition, given the patient’s peripheral smear showing spherocytosis, G6PD levels were also assessed and found to be low consistent with mild to moderate G6PD deficiency. The patient’s family was educated about precautions to take to reduce the risk of excessive oxidative stress that could precipitate acute haemolytic anaemia episodes in the future.
Glucose-6-Phosphate-Dehydrogenase (G6PD) levels are shown in Table 1 and Haemoglobin Electrophoresis results are shown in Table 2. Overview of Hemoglobinopathies is shown in Figure 1.
| Type | Units |
|---|---|
| G6PD | 60 units/trillion RBCs |
Table 1: Glucose-6-Phosphate-Dehydrogenase (G6PD) levels.
| Hemoglobin type | Results |
|---|---|
| A2 | 0% |
| F | 79.3% |
| A | 14.2% |
| S | 6.5% |
| Hb Barts | Trace |
Table 2: Haemoglobin electrophoresis results.
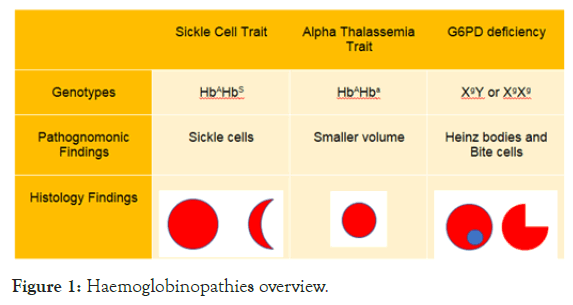
Figure 1: Haemoglobinopathies overview.
Genotype for Sickle Cell Trait is HbAHbS
Genotype for Alpha Thalassemia Trait is HbAHba
Genotype for G6PD deficiency is XgY or XgXg
Where A=normal haemoglobin variant, S=Sickle cell trait, a=alpha thalassemia trait, g=G6PD deficiency trait.
The infant was seen again 6 months later in clinic and has remained asymptomatic without any other hospitalizations since initial evaluation.
The Centers for Disease Control (CDC) estimates that approximately 1.5% of newborns are positive for sickle cell trait in the United States [1]. While most of these infants are asymptomatic and rarely require hospitalization during their lives, co-occurrence of alpha thalassemia trait and G6PD deficiency may have mixed effects on a patient’s susceptibility to hemolytic anemias. Some studies of patients with both G6PD deficiency and sickle cell disease do not demonstrate an increased risk for pain crises, but other studies appear to show that hemolytic anemia episodes when they do occur may last longer than usual [4,5]. There is less evidence of increased morbidity in patients who are only carriers of the sickle cell trait and have G6PD deficiency [6]. In general however, both conditions are treated separately in most patients and no special measures are taken when managing both conditions concurrently.
In contrast to G6PD deficiency, it is theorized that alpha thalassemia may actually have a protective effect in patients with sickle cell trait [7]. Studies of patients with both alpha thalassemia and sickle cell trait have often demonstrated lower concentrations of hemoglobin S in erythrocytes leading to lower rates of hemolytic anemia overall [7]. This is largely due to elevated levels of hemoglobin F compared to hemoglobin S in patients who have sickle cell trait alone [7]. Possibly as a result of this, some studies have shown that patients with alpha thalassemia and sickle cell trait often have lower rates of hyposthenuria, hematuria, and chronic kidney disease [8,9].
There are almost no documented cases of concurrent alpha thalassemia, sickle cell trait, and G6PD in the literature but given the prevalence of each trait across similar populations it may be more common than has been recorded. Each of the three traits is thought to contribute protection against malaria, albeit in different ways. The exact mechanism by which alpha thalassemia protects against malaria is unclear, but theories include increased antibody binding to alpha thalassemia RBCs infected with plasmodium parasites, as well as alpha thalassemia patients having a larger number of red blood cells to make up for their diminished RBC volumes, thus enabling them to better overcome a malarial infection [10,11].
The prevalence of sickle cell disease in malaria endemic regions is well documented and has long been associated with protection against Plasmodium falciparum. The mechanism of protection, especially in patients who are only carriers of the sickle cell trait, is less understood however. The most popular theories hypothesize that Plasmodium falciparum does not grow as readily in HbAS RBCs, that RBCs infected by the parasite sickle and get removed by the spleen before more RBCs can get infected, and that HbAS may enhance innate immunity to parasitic RBC infection [12]. G6PD deficiency, similarly, is thought to help protect hosts from malaria due to the destruction of infected RBCs from the additional oxidative stress of parasitic infection before the parasite can grow and spread to other cells. Despite the unique mechanisms by which, each genetic condition affects RBC stability, it is unclear how much sickle cell trait, alpha thalassemia, and G6PD deficiency interact to cause hemolytic anemia. Current practice is to treat each condition separately and it is unclear whether there is a multiplier effect from having all three conditions at the same time.
The concurrent presentation of sickle cell trait, alpha thalassemia, and G6PD deficiency is rare and it is theorized that each trait respectively confers an evolutionary advantage against malaria. While the exact prevalence of alpha thalassemia and G6PD deficiency in the U.S is not well documented, each genotype often co-presents with sickle cell trait individually and should be considered in patients with persistent neonatal hyperbilirubinemia secondary to acute haemolytic anaemia.
Citation: Krupadev V, Kirbens J, Rafique A (2021) A Case of Concurrent Sickle Cell Trait, Alpha Thalassemia, and G6PD Deficiency in a Pediatric Patient. J Cancer Res Immunocol. 7:129.
Received: 31-Dec-2020 Accepted: 14-Jan-2021 Published: 21-Jan-2021 , DOI: 10.35248/2684-1266.21.7.129
Copyright: © 2021 Krupadev V, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.