
Journal of Clinical Toxicology
Open Access
ISSN: 2161-0495

ISSN: 2161-0495
Case Report - (2021)Volume 11, Issue 4
Fluoroacetate poisoning, usually caused by exposure to sodium monofluoroacetate and fluoroacetamide, competitively inhibits aconitase in the tricarboxylic acid cycle. There are no effective antidotes, and treatment is symptomatic and supportive. The effectiveness of hemodialysis in alleviating fluoroacetate poisoning remains unclear, and no study has reported changes in the blood concentration of fluoroacetate after hemodialysis. A 71-year-old man with a history of chronic kidney disease and on maintenance hemodialysis had findings suggestive of acute fluoroacetate poisoning. He presented with tachycardia, tachypnea, renal dysfunction, hyperammonemia, and hyponatremia. He was intubated with a nasogastric tube and administered intravenous fluids, and direct hemoperfusion and hemodialysis were performed on successive days to reduce blood fluoroacetate concentrations. He recovered without organ failure and was transferred for rehabilitation. This is the first study to report a case wherein treatment with hemodialysis reduced fluoroacetate concentrations, suggesting hemodialysis can be an effective treatment strategy for fluoroacetate poisoning.
Aconitase; Dialysis; Fluoroacetamide; fluorocitrate; Tricarboxylic acid cycle
Fluoro Acetate (FA) poisoning is caused by exposure to Sodium Mono Fluoro Acetate (SMFA) and Fluoro Acet Amide (FAA). SMFA (compound 1080) is used as a rodenticide in the United States, Australia, and New Zealand [1,2]. FAA (compound 1081) is a rodenticide with moderate-to-high toxicity. In Japan, FAA was used as an insecticide in orchards in the 1960s; however, the widespread use of organophosphorus pesticides led to the withdrawal of the registration of FAA in 1975. The highly toxic nature of these compounds necessitated a license for their manufacturing, ownership, and usage in many countries. Therefore, cases of FA poisoning have become uncommon in recent years. However, there are recent reports of FA poisoning from China because of its market availability, low cost, and high efficiency.
Studies have described the toxicological mechanisms underlying FA poisoning. FA causes cytotoxicity by competitively inhibiting aconitase in the Tri Carboxylic Acid (TCA) cycle. There are no effective antidotes for FA poisoning, and symptomatic treatment is the mainstay of therapy [3]. FA poisoning is characterized by a short latent period (0.5–6 hours) between ingestion and symptom onset. Hence, prompt reduction of FA concentrations in the bloodstream is important. Acute Blood Purification (ABP) is not included in the usual treatment for FA poisoning; however, it has been reported in some studies from the United States and China. Furthermore, the use of Hemo Dialysis (HD) in rapidly reducing FA concentrations and alleviating FA poisoning remains unclear, and no study has reported changes in blood FA concentrations after HD.
Herein, we report a case of FAA poisoning in a patient on maintenance dialysis, in whom FA poisoning was alleviated using HD.
A 71-year-old man with a history of chronic kidney disease, chronic heart failure, alcoholic hepatitis, and schizophrenia and on maintenance HD was found unconscious in his home by his family at 1:10 PM, and an emergency call was made. There was vomit and an empty 500-mL bottle in the vicinity. The contents of the bottle included 10% FAA, 90% water, and dye. A doctor helicopter was called in because it was a case of acute poisoning. The examination at 1:47 PM. Noted that the patient was unconscious (Glasgow Coma Scale score: E4V1M5), tachycardic (heart rate: 103 beats/min), and tachypneic (respiratory rate: 40 breaths/min). His systolic blood pressure was 132 mmHg, and oxygen saturation by pulse oximetry was 100% (O2 at 4 L/min). He was intubated with a nasogastric tube and administered intravenous fluids. Furthermore, 400 mL of blue fluid was drained from his stomach. On arrival at our hospital at 2:29 PM, the patient’s cardiovascular and respiratory parameters had stabilized because of sedation. Physical examinations revealed a temperature of 36.1°C, blood pressure of 116/71 mmHg, heart rate of 78 beats/min, and respiratory rate of 20 breaths/min; other findings were unremarkable. Laboratory investigations revealed renal dysfunction (creatinine, 685.1 μ mol/L; blood urea, 18.6 mmol/L), hyperammonemia (489.1 μ mol/L), and hyponatremia (125 mmol/L). Arterial blood gases showed metabolic acidosis (pH, 7.259; pCO2 , 17.9 mmHg; pO2 , 302.0 mmHg; HCO3, 7.7 mEq/L;and lactate [Lac], 3.3 mmol/L). Computed tomography showed no cerebral hemorrhage; however, mild bilateral pneumonia was noted. After gastric lavage and activated charcoal administration, he was admitted to the emergency intensive care unit. We performed direct hemoperfusion (DHP; quantity of blood flow: 100 mL/min) using a coated activated charcoal column (Hemosorba CHS-350; Asahi Kasei Medical, Tokyo, Japan) because paraquat poisoning was suspected based on the drained blue fluid. In addition, DHP and HD (quantity of blood flow: 200 mL/min) using a dialyzer (VPS-18HA; Asahi Kasei Medical, Tokyo, Japan) were performed with the administration of heparin sodium at approximately 4.5 and 6 hours after ingestion, respectively. Similarly, we measured the blood FA concentration in a stepwise manner before and after DHP and HD (Figure 1).
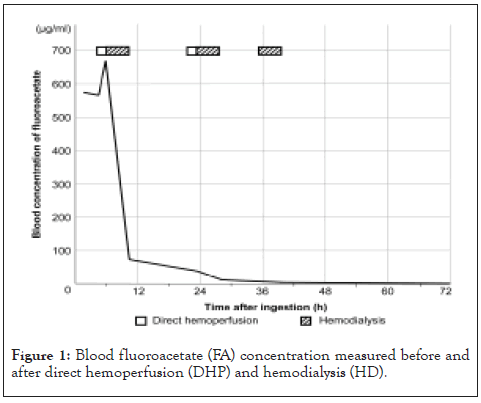
Figure 1: Blood fluoroacetate (FA) concentration measured before and after direct hemoperfusion (DHP) and hemodialysis (HD).
FA was extracted using solid-phase micro extraction, and analyses were performed by gas chromatography-mass spectrometry. The blood FA concentrations were 578.1 μg/mL on arrival at the hospital (1.5 hours after ingestion) and increased to 674.8 μg/mL after DHP (6 hours after ingestion). After HD, FA concentrations markedly decreased to 75.2 μg/mL (10 hours after ingestion). The Lac concentrations were 1.3 mmol/L and 1.8 mmol/L after initial DHP and HD, respectively. After confirming that paraquat was undetectable, we terminated the DHP after two sessions and continued with HD. Blood FA and Lac concentrations decreased to 4.3 μg/mL and 0.7 mmol/L, respectively, after three HD sessions (39 hours after ingestion). We extubated him on day 8 of hospitalization after improvement in the symptoms of aspiration pneumonia. There were no indications of organ damage, and only minor cognitive impairment was observed. He was transferred for rehabilitation on day 22 of hospitalization.
Recent reports of FA poisoning have been mostly reported from China [4]. However, there are few studies on FA poisoning in humans; therefore, detailed information is limited. FAA has a low molecular weight (77.06 g/mol), no odor or flavor, and is freely soluble in water. The lethal doses of FAA and FA in humans are 2–5 and 2–10 mg/kg, respectively. The half-life of fluoroacetate is species dependent. The reported half-lives in rabbits, goats, and sheep are 1.1, 5.4, and 10.8–11 h, respectively [5]. Notably, in this report, we only searched for literature that was written in English.
FAA is hydrolyzed to FA after administration, similar to SMFA. Subsequently, it is metabolized to fluorocitrate (FC) in the TCA cycle, which competitively inhibits aconitase (Figure 2).
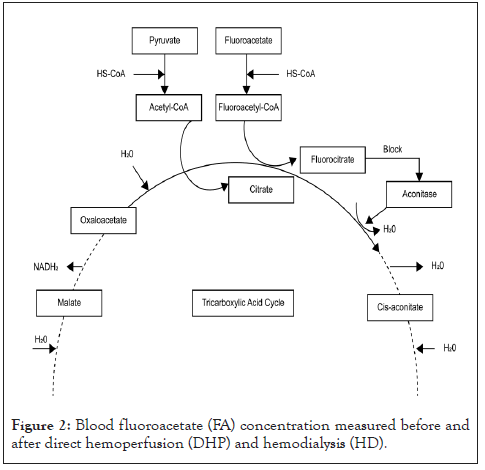
Figure 2: Blood fluoroacetate (FA) concentration measured before and after direct hemoperfusion (DHP) and hemodialysis (HD).
This process is called “lethal synthesis” [6,7]. Consequently, the suppression of the TCA cycle in the mitochondria impairs aerobic metabolism, thus causing cytotoxicity. The direct or indirect impairment of oxidative metabolism leads to the development of symptoms related to FA poisoning. These include convulsions, loss of consciousness, cardiac rhythm disturbances (tachycardia, bradycardia, prolonged QT, ventricular fibrillation, hypotension due to decreased peripheral vascular resistance, electrolyte abnormalities (hypocalcemia and hypopotassemia), acute renal failure, and metabolic acidosis. The clinical pattern of FA poisoning characteristically involves a latent period of 0.5–6 hours. The latent period depends on the hydrolysis of FA salts to monofluoroacetic acid, synthesis of effective FC concentrations, and disruption of intracellular processes. FAA and SMFA are metabolized by the body and excreted in urine and stool. In the current case, FAA poisoning occurred in a patient with a pre-existing impairment in renal function; consequently, we anticipated irreversible and fatal damage.
Although the mechanism underlying FA poisoning is understood, there is no antidote that is superior to symptomatic and supportive treatment. The most accepted treatment is the administration of ethanol immediately after poisoning [8]. However, this is seldom followed because the effectiveness of ethanol has been reported mostly in experimental animal studies, and it is difficult to administer immediately after FA poisoning owing to clinical signs similar to those of other poisonings. ABP is not included in the general treatment for FA poisoning; however, the efficacy of DHP against FAA has been reported in studies from China.
Nevertheless, there is also a report that DHP is ineffective [9]. In our case, we measured the FA blood concentrations before and after HD and DHP. Although blood concentrations of FA could not be confirmed in a timely manner, we presumed that the significant decrease in blood concentrations of FA following HD indicated that HD was effective. FAA and FA are substances that can be removed by HD because of their low molecular weight and high hydrophilicity. However, because FA is a compound and its usage is legally restricted in Japan, we consider it a limitation that we have not been able to investigate the distribution volume and protein binding ratio of FAA and FA. Clinically, FA is a poison that causes irreversible damage with the rapid onset of symptoms, but the patient did not show cytotoxicity after the initial HD. Furthermore, the survival of the patient indicates that HD was useful.
The patient, a 74-year-old man with pre-existing renal impairment, survived potentially fatal FAA poisoning without significant sequelae after the timely administration of HD following the ingestion of the toxic compound. In this case, HD significantly lowered blood concentrations of FA, thus preventing the ongoing suppression of the TCA cycle and resultant cytotoxicity. This is the first case wherein HD treatment reduced the blood concentration of FA. However, comparative studies are necessary to validate the findings. We hope that the findings of this case will contribute to the treatment of FA poisoning.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. We would like to thank Editage for the English language editing.
Citation: Higami S, Morita S, Sugita M,Yamamoto R, Saito T, Nakagawa Y (2021) A Case of Fluoroacetamide Poisoning Alleviated by Hemodialysis. J Clin Toxicol. 11:477.
Received: 02-Mar-2021 Accepted: 16-Mar-2021 Published: 23-Mar-2021 , DOI: 10.35248/2161-0495.21.11.477
Copyright: © 2021 Higami S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.