Journal of Antivirals & Antiretrovirals
Open Access
ISSN: 1948-5964
ISSN: 1948-5964
Research Article - (2021)Volume 13, Issue 3
Objective: SARS-CoV disease caused by SARS-CoV-1 is of multifactorial etiology consisting of upstream and downstream phases. The upstream phase is the physical breach of the cells protective shield by extraneous stressors including the virus and environmental elements (xeno), while the downstream is a sequela of damages (plexic) manifested in various symptoms, herein called xenoplexic diseases. Symptom targeting drugs are ineffective and palliative at best. SARS-CoV is a xenoplexic disease best treated with a combo therapeutic strategy This study evaluates EmbotricinTM (EMB), a multicomponent compound, to treat SARS-CoV.
Materials and methods: Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV-1), strain Urbani (200300592), obtained from the Centers for Disease Control and Prevention (CDC, Atlanta, GA) was used as the test virus. Female BALB/C mice obtained from Charles River Laboratories (Wilmington, MA) were the animal host. New Chemical Entities (NCE) including FTX-214, FTX-218, and FTX-219 were formulated as a 3-component combo, herein called Embotricin (EMB). Also, a 4-component compound consisting of EMB plus sodium thiosulfate was evaluated. The 3- and 4-combo compounds were administered 2 days prior to infection, given by per os (PO) daily (q.d.) for 5 days. Poly I:C, a known antiviral compound acting as an immunomodulator was used as a comparative control, administered intraperitoneally (IP).
Results: EMB at 3.1 mg/kg fixed dose (1:1:1 ratio) combo reduced viral load by 14.4% compared to 11.8% for poly I:C at 10 mg/kg dose. Addition of sodium thiosulfate (4-combo formulation) further boosted the activity to 16.6%. Increasing the 4-combo dosages to 4.65 mg/kg and 6.2 mg/kg further increased activity to 18.2% and 20.9%, respectively.
Conclusion: The individual components synergistically targeted the upstream disease etiology and restore in toto the health of the cell to ward off SARS-CoV-1. Thus, combo therapy may well be a universal platform to treat xenoplexic diseases where monotherapies fail. The combo therapy effectively reduced the viral load of SARS-CoV-1, not by directly inhibiting the virus, but most likely due to enhancing the health of the cell.
Xenoplexic disease; Combo therapy; SARS-CoV-1; Glycocalyx; Reactive Oxygen Species (ROS); Particulate Matters (PMs); ACE2 receptors; Heparan sulfate; Cellular-humoral immunity
Majority of diseases are triggered by a breach in the cell’s protective layer called Glycocalyx (GCX). The breach is caused by extraneous factors including lifestyle (stress, physical and mental abuse, diet, drug usage) and environmental stressors (toxins, pathogens, radiation, chemicals pollutants in foods, personal care products, household goods, air soil) [1,2]. The breach is the upstream cause of many diseases including infections, inflammatory, neurological, immunological, hematological, endocrine disorders, cancer, macular dysfunction, and the family of Cardiovascular Disease (CVD). If the breach is not repaired, damages cascade downstream and manifested as disease symptoms. Although only a minority of diseases are of true monogenic genetic origin, they instilled the ‘one gene-one enzyme’ and ‘one drug-one-target concept of drug discovery and inspired the Human Genome project, which promised some 30,000 genes as “druggable” targets [3,4]. However, finding the target gene for a ‘magic-bullet’ has proven to be unsuccessful because >90% of diseases are of extraneous etiology with genetic factors account for only a minority [5,6].
Human cells are organized in 4 tissues including epithelium nervous, muscle and connective tissue. Epithelium covers body surfaces, lines internal closed cavities including glands, body tubes and the vascular system. Epithelial tissues protect underlying tissues from radiation, desiccation, toxins, pathogens, and physical trauma; regulate exchange of chemicals between tissues and a body cavity; secrete hormones into the blood vascular system, provide sensation. A form of epithelium are Endothelial Cells (EC), which line the internal surface of the circulatory system including the lumen of the arteries, veins, lymphatic vessels, blood capillaries and cavities of the heart. Yet another layer, the GCX, coats the epithelial and endothelial cells providing the first line of protection from physical, chemical, and biological wear and tear and acts as a fluid barrier and regulates flow of erythrocytes [7-9].
Glycocalyx is a fuzz-like carbohydrate-rich coating of 0.3-0.6 μm thick that covers the membrane of ECs and excessive shedding of GCX triggers diseases [10,11]. The infectious agent SARS-Cov-1, responsible for the 2002-2003 severe acute respiratory syndrome coronavirus (SARS-CoV) pandemic, was one of the most significant public health events and the current ongoing SARS-CoV-2 infection as one of the deadliest pandemics (COVID-19) in history [12,13]. SARS Cov-1 is a strain of virus similar to SARS-cov-2 which is an enveloped, positive-sense; single-stranded RNA virus that breaches the GCX and infects the lung epithelial cells [14-16].
A key to the success of developing therapies for these viruses is understanding disease etiology and identify “druggable” targets. Thus, infection starts through a disruption in the EC lining and then both SARS CoV-1 and SARS-CoV-2 binds to ACE2 Receptor Binding Domain (RBD) proteins [17,18]. All viruses take entry by breaching or taking advantage of a disrupted EC [19] For example, GCX shedding was observed in newly intubated COVID-19 patients with imminent infection [20].
In perspective, the first line of defense to respiratory infections is the negatively charged mucus (mixture of water, epithelial cells, dead leukocytes, mucin, and inorganic salts) secreted by the nasal epithelium. The mucus hydrates the GCX-Periciliary Layer (PCL) to maintain Na+/Cl- ion balance, lubricates ciliary beating, restricts particles to cell lumen and traps noxious agents [21-23]. The negatively charged mucus glides over the negatively charged GCX- PCL for clearance and disposed via swallowing or expectoration (sneeze, cough, spit) [24,25]. Air pollutants, particularly Particulate Matters (PMs) including persistent organic pollutants, volatile and semi-volatile organic compounds, disrupt the Na+ and Cl- ion balance resulting in a loss of PCL volume and composition [26]. Moreover, the damaged GCX-PCL generates extraneous Reactive Oxygen Species (ROS), which further decrease Mucociliary Clearance (MCC) and disorientation of cilia [27-29]. Indeed, PMs have been reported to exacerbate severity of COVID-19 in England, northern Italy and SARS-CoV in China [30-32]. In the US, PM elevation of 1 μg/m3 equates to 15% increase in death rates and chronic PM 2.5 exposure significantly increase COVID-19 mortality [33,34].
At the molecular level, PMs ‘steal’ electrons from the GCX shield creating oxidative damage, which generate debris or detritus that are antigenic. Antigenic debris trigger release of histamine, which binds to various cellular debris resulting in inflammation, additional ROS, and cell distress [35]. In distressed tissues, histamine determines the fate of the cell with three options: apoptosis, autophagy, or necrosis, and inadequate repair leads to various diseases [36]. On the other hand, viruses are bioactive pollutants.
Normally, the negatively charged GCX repels the negatively charged SARS-CoV virus, but a breach in GCX shield allows viral entry [37]. Thus, the Hemagglutinin Esterase (HE) glycoprotein in the viral surface of SARS-CoV destroys the negative GCX shield to start infection [38]. While PMs are oxidative and generate cellular debris, viruses on entry are tagged by Recognition Receptors (PRR) e.g., Toll-like Receptors (TLRs), which activates neutrophil and digest the virus into debris (cellular immunity). Viral debris activates the classic M1 macrophage by binding with CD4 glycoprotein on to CD4 T helper cells releasing IL-12 cytokine and interferon IFN-Υ resulting in cytokine/ROS storm and cell death [39]. Concomitantly, the humoral immunity is activated with the CD4 T helper cells binding to B cell (CD19), which produce IL-2, -4, -5 and activate plasma cells to produce the antibodies (Abs), IgM, IgB, IgA, IgE. These antibodies activate the regulatory macrophage (M2), which decrease inflammation and encourage tissue repair [40]. CD8 T-helper cells effectively decrease viral load in SARS patients and persist for up to 6 years while B cell (CD19) are short- lived [41,42]. Thus, regulating oxidative/inflammatory effect of CD8/M2 is more effective than Ab therapy. The manifestation of viral infection on cellular and humoral immunity is shown in Figure 1.
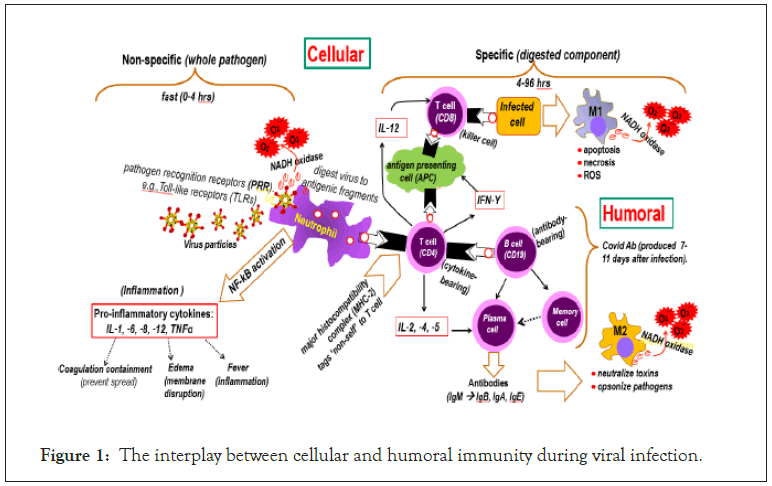
Figure 1: The interplay between cellular and humoral immunity during viral infection.
The nose contains the highest percentage of ACE2 receptors to which the virus micro-aspirates into deep lung (gastro-esophageal reflex) at night and subsequently spreads downstream to the ACE2 of other organs: Highest levels in small intestine, testis, kidneys, heart, thyroid, adipocytes; medium levels in lungs, colon, liver, bladder, adrenal gland; and, lowest in blood, spleen, bone marrow, brain, blood vessels, muscle [43-45].
The traditional “one drug-one target-one disease" discovery paradigm does not address the multifactorial etiology of SARS- CoV infection. SARS-CoV is a disease that includes two phases: An upstream physical breach of the cells protective shield, and a downstream manifestation of infection. The upstream are extraneous factors including the virus and environmental stressors (xeno), while the downstream is a sequela of damages (plexic) manifested in various symptoms, herein called xenoplexic diseases. Symptom targeting drugs are ineffective and palliative at best. SARS-CoV is a xenoplexic disease best treated with a combo therapeutic strategy. This study evaluates EmbotricinTM (EMB), a multicomponent compound, to treat SARS-CoV.
Drug targets and synthesized compounds
The multimodal etiology of SARS include disruption of glycocalyx shield and a sequela of cellular and humoral activation involving oxidative and inflammatory damages. With this backdrop, several New Chemical Entities (NCE) designated as FTX compounds, were synthesized to individually address GCX repair and mitigation of oxidative and inflammatory damages (Figure 2).
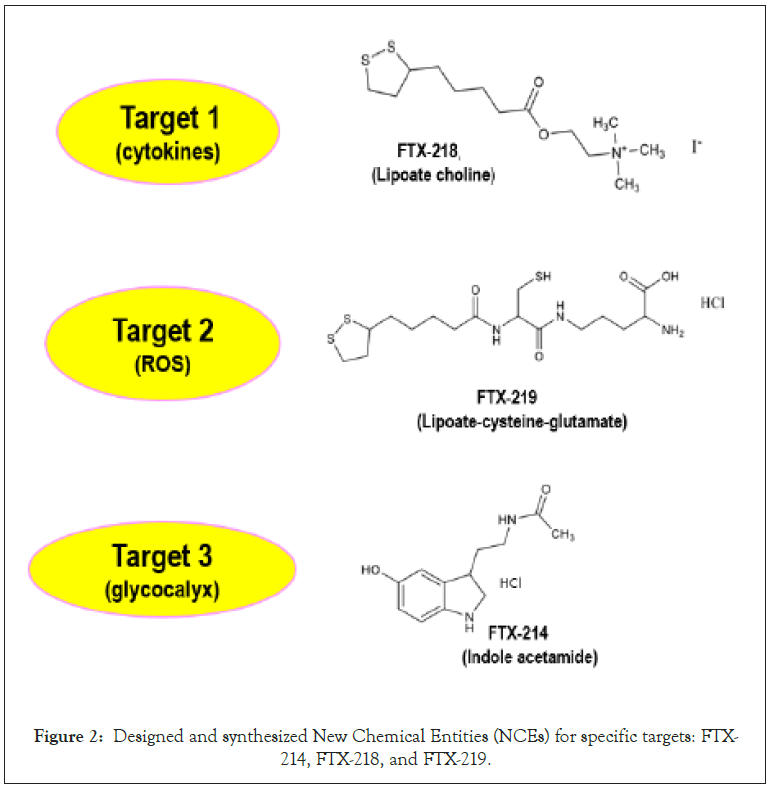
Figure 2: Designed and synthesized New Chemical Entities (NCEs) for specific targets: FTX- 214, FTX-218, and FTX-219.
Synthesis of these NCEs was based on empirical knowledge of active drug scaffolds and mode of action to address the indicated targets. Since no single NCE could address in toto the xenoplexic nature of SARS a combo-compound therapy was the best option. Thus, an abbreviated 3-factor permutation combination was carried out on the NCEs on an animal model which yielded an optimum fixed- dose 1:1:1 ratio combo of FTX-214, -218, and -219 [46].
Virus
Severe Acute Respiratory Syndrome-Associated Coronavirus (SARS-CoV-1) Urbani strain was obtained from the Centers for Disease Control and Prevention and adapted for lethality in mice as described [47]. Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV-1), strain Urbani (200300592), was obtained from the Centers for Disease Control and Prevention (CDC, Atlanta, GA) and routinely passaged in Vero-76 (ATCC CRL1587) cells [15]. Virus titration: Animal was euthanized, and the lungs were removed. Lungs were then diluted 1:20 in MEM +10% FBS, homogenized, and held at -80°C for virus titer testing. Lung homogenates were later thawed and serially diluted in MEM +2% FBS in triplicate in 96-well plates containing Vero-76 cells. Plates were incubated for 6 days and then checked for virus-induced Cytopathic Effect (CPE) [48]. Quantitation of virus yield titers was by the end point method using the Cell Culture 50% Infectious Dose (CCID50) assay [49].
Animals
Female 5-week-old BALB/C mice were obtained from Charles River Laboratories (Wilmington, MA) for this experiment. The mice were quarantined for 3 days before use and maintained on Teklad Rodent Diet (Harlan Teklad) and tap water at the Laboratory Animal Research Center of Utah State University.
Experiment design
Per experimental design laid out in Table 1 a total of 80 mice were randomized into 4 groups of 8 mice per group. The compounds were solubilized using in vehicle comprised of 20% cremophor and 80% saline.
| Study segments | dose (mg/kg/day) |
|---|---|
| 3-combo component: FTX-214/FTX-218/FTX-219 | |
| A. 1.0:1.0:1.0 ratio | 3.1 |
| B. 0.5:1.5:1.0 ratio | 3.1 |
| C. 1.0:0.5:1.5 ratio | 3.1 |
| D. 1.5:1.0:0.5 ratio | 3.1 |
| 4-combo component: FTX-214/FTX-218/FTX-219/Na thiosulfate | |
| E. low dose | 1.55 |
| F. Normal dose | 3.1 |
| G. Medium dose | 4.65 |
| H. High dose | 6.2 |
| Reference Compounds | |
| I. Poly (I:C) | 10 |
| J. Placebo (cremophor/saline) |
Table 1: Study layout including formulation ratio of component compounds and doses.
Treatment with compounds
A proven antiviral drug poly I:C was used as a positive control for this study. Poly I:C is a synthetic dsRNA polymer that induces interferon production (Figure 3) [50,51].
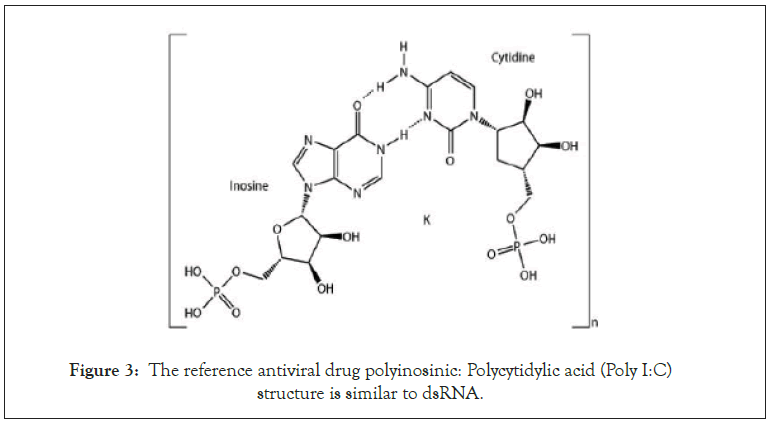
Figure 3: The reference antiviral drug polyinosinic: Polycytidylic acid (Poly I:C) structure is similar to dsRNA.
The compounds were administered 2 days prior to infection, given by per os (PO) daily (q.d.) for 5 days. Poly I:C was given twice daily (b.i.d.) by IP administration beginning 1 day prior to infection. For challenge, mice were anesthetized by IP injection of ketamine/ xylazine (50 mg/kg/5 mg/kg) prior to challenge by the intranasal route with a dose of 1 × 10 3.5 50% cell culture infectious doses (CCID50) in a 90 μl inoculum volume. Four (4) mice sacrificed at days 3 and 5 post-infection for lung virus titers.
Ethics regulation of laboratory animals
This study was conducted in accordance with the approval of the Institutional Animal Care and Use Committee of Utah State University dated March 31, 2020 (expires March 30, 2023). The work was done in the AAALAC accredited Laboratory Animal Research Center of Utah State University. The US Government (National Institutes of Health) approval was renewed March 9, 2018 (PHS Assurance No. D16-00468[A3801-01]) in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (Revision; 2011).
Virus lung titers
The first segment of this study (I) evaluated different ratios of the 3 FTX base EMB components in the following sequence FTX-214/ FTX-218/FTX-219: (A) 1.0:1.0:1.0; (B) 0.5:1.5:1.0; (C) 1.0:0.5:1.5 ratio; and (D) 1.5:1.0:0.5. A second segment evaluated the basic EMB with thiosulfate added (Figure 4).
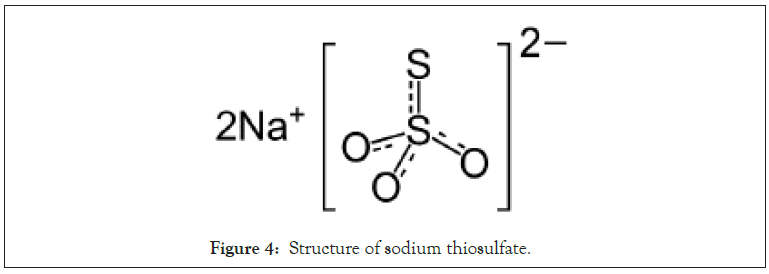
Figure 4: Structure of sodium thiosulfate.
Thus a 4-combo component with the ratio of 1:1:1:1 (FTX-214/ FTX-218/FTX-219/sodium thiosulfate). The 3rd segment is the incorporation of a known antiviral drug Poly I:C as a comparative positive control, and an untreated control or placebo (Table 1).
The virus titers in all the mice are shown in Table 2.
| Study segments | Lung virus titer: CC ID/50 (Log 10) | |||||||
|---|---|---|---|---|---|---|---|---|
| Day 3 | Day 5 | |||||||
| Mouse 1 | Mouse 2 | Mouse 3 | Mouse 4 | Mouse 1 | Mouse 2 | Mouse 3 | Mouse 4 | |
| 3-combo component (FTX-214/FTX-218/FTX-219) | ||||||||
| A. 1.0:1.0:1:0 ratio (3.1 mg/kg/d) | 8 | 8 | 8.2 | 7.5 | 6.7 | 7.5 | 7 | 6.2 |
| B. 0.5:1.5:1.0 ratio (3.1 mg/kg/d) | 8 | 8.2 | 8.2 | 7.2 | 7 | 7.2 | 7.2 | 6.5 |
| C. 1.0:0.5:1.5 ratio (3.1 mg/kg/d) | 7.2 | 8.2 | 8 | 6.2 | 6.2 | 7.2 | 7.2 | 7 |
| D. 1.5:1.0:0.5 ratio (3.1 mg/kg/d) | 8.2 | 8 | 8.2 | 7.2 | 7.2 | 7.2 | x | 6.7 |
| 4-combo component (FTX-214/FTX-218/FTX-219/thiosulfate) | ||||||||
| E.low dose (1.55 mg/kg/d) | 7.5 | 7.5 | 7.5 | 7.2 | 6.2 | 7.2 | 7.5 | 7.2 |
| F. Normal dose (3.1 mg/kg/d) | 7.5 | 8.2 | 8 | 7.5 | 6.2 | 7 | 6.2 | 6.7 |
| G. Medium dose (4.65 mg/kg/d) | 7.5 | 8.2 | 7.2 | 8.2 | 6.2 | 6.2 | 7.2 | 5.7 |
| H. High dose (6.2 mg/kg/d) | 8.2 | 8.2 | 8 | 8.2 | 6.2 | 6.2 | 6.2 | 7 |
| Reference compounds | ||||||||
| I. Poly (I:C) (10.0 mg/kg/d) | 7.2 | 7.2 | 7 | 7.2 | 6.5 | 6 | 6.5 | 6.2 |
| J. Placebo (cremophor/saline) | 8.2 | 8.2 | 8.2 | 8.2 | 8 | 7 | 7 | 8 |
Table 2: Lung virus titers from BALB/c mice treated with 3-and 4-combo EMB.
All the treatments significantly (**P<0.01) reduced lung virus titers on day 5 post-infection compared to placebo (Figure 5).
Figure 5: Treatments A-I significantly reduced SARS-CoV lung virus titers on day 5 post-infection compared to placebo (**P<0.01).
Efficacy of the combo treatments in reducing viral load
The mice were sacrificed at days 3 and 5 post-infection for lung virus titers. The average virus titer levels of the 4 mice in each group were calculated and the reduction of virus titers from the 3-day to the 5-day mark is used to evaluate antiviral efficacy. Thus, the more negative the value, the better the activity. For example, treatment H has the best activity with a value of -1.7 (Figure 6).
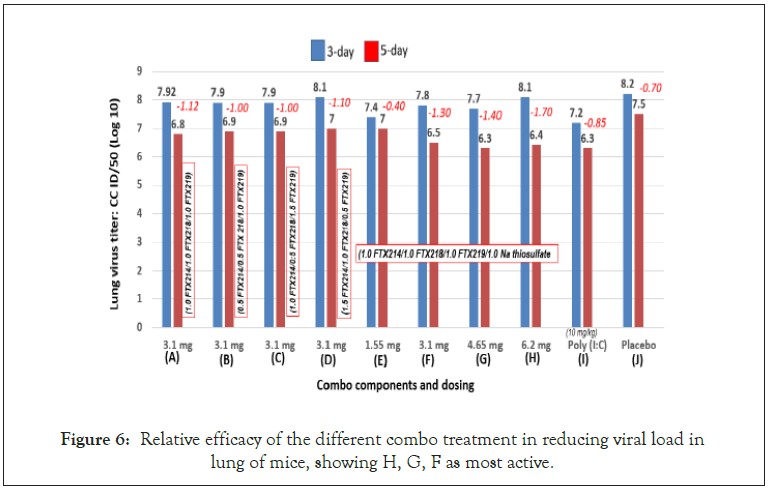
Figure 6: Relative efficacy of the different combo treatment in reducing viral load in lung of mice, showing H, G, F as most active.
Efficacy of the combo calculated as percent reduction in viral load
Another metric for evaluating efficacy is by percent reduction in viral load. Thus, the difference between 3-day and 5-day was divided by the 3-day value and expressed as percent. With this metric the order of efficacy is as follows: H>G>F>A>D>B=C>I>J>E (Figure 7). Note that all the combos, except the low dose (1.55 mg/kg) were of superior activities over the antiviral control Poly (I:C).
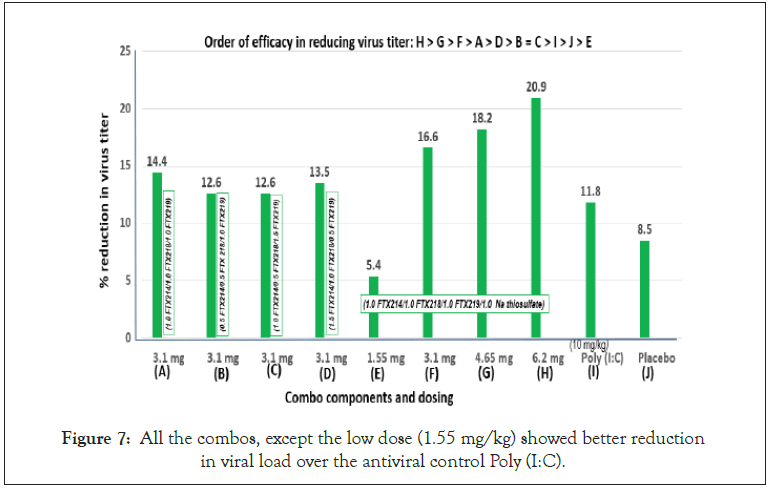
Figure 7: All the combos, except the low dose (1.55 mg/kg) showed better reduction in viral load over the antiviral control Poly (I:C).
In this study Poly I:C was administered 1 day prior to infection to build up adaptive immunity. As a parallel comparison, EMB and combo compounds were also administered a day prior to infection. As expected, Poly I:C-treated mice showed lower viral titers in the 3-day mark than EMB or the combo compounds, which confirms the potent immunogenic action of Poly I:C (Figure 8) [52,53].
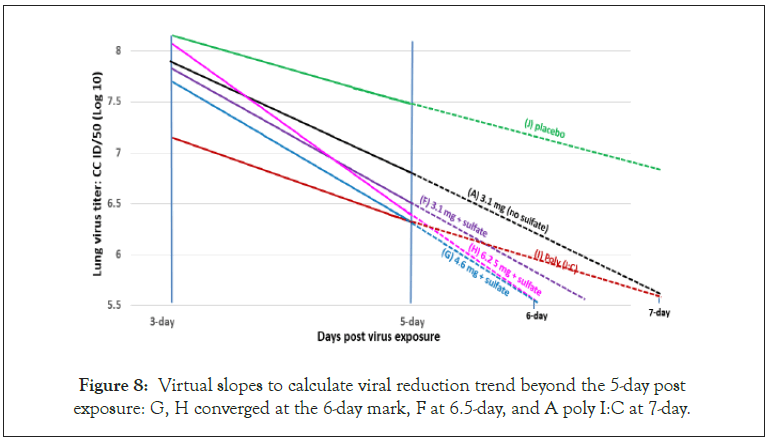
Figure 8: Virtual slopes to calculate viral reduction trend beyond the 5-day post exposure: G, H converged at the 6-day mark, F at 6.5-day, and A poly I:C at 7-day.
However, as treatment continued, the rate of viral reduction slowed down in the Poly I:C treated mice, while the EMB compounds showed sharp reduction in viral load, for example 11.8% reduction in Poly I:C compared to 20.9% for combo compound H (Figure 7).
The basic EMB formulation is a fixed dose combo of FTX-214/ FTX-218/FTX-219 at 1:1:1 ratio. To evaluate the importance of each component, a brief factorial study was carried out by changing ratios of the components in the combo. Figure 5 shows that changing the ratio did not improve the activity over the 1:1:1 ratio, which indicates equal contribution of the components, the 3 components were synergistically confirming previous results in another disease indication [46].
Projected efficacy slopes
There were significant reductions in viral load in all the treatments, albeit calculated from two points. In this regard, virtual linear slopes were calculated beyond the 5-day post infection. The best slopes were G (6.2 mg EMB +SO4) and H (6.2 mg EMB+SO4), which converged at the 6-day mark, then F at 6.5-day mark followed by I (poly I:C) and A (EMB, no thiosulfate) and poly (I:C) converged at the 7-day mark (Figure 8).
Sodium thiosulfate boosted viral reduction
The outermost component of the GCX is Heparan Sulfate (HS) and the loss of sulfate disrupts the integrity of the heparan sulfate shield accounting for viral entry [54,55].
In a preliminary study (unpublished), addition of sodium thiosulfate to the 3-combo compound enhanced the activity of EMB. This study confirmed such result, showing that adding thiosulfate to EMB as a 4th component to create a 4-combo compound (FTX- 214/FTX-218/FTX219/sodium thiosulfate) at 1:1:1:1 boosted the activity of EMB (Figure 6).
Further, 4 dosages of the fixed-dose formulation were evaluated including low (1.55 mg), control (3.1 mg), medium (4.65 mg) and high (6.2 mg). A linear dose response was observed with 1.55 mg as the least active (5.4% viral load reduction) and 6.2 mg as the highest with 20.9% viral load reduction (Figure 7).
Per xenoplexic disease concept, etiologies are divided into two segments: upstream and downstream. In SARS-cov, upstream is represented by the physical barriers including mucus through the GCX-PCL shield. Once these physical barriers are breached the virus gain entry, which turn on the adaptive immunity including cellular and humoral.
Best treatment strategies target the upstream etiology. As cell damages cascade downstream, manifestations show as symptoms. Current drug development strategies are a monotherapy platform based on the ‘one gene-one disease’ paradigm, targeting a specific symptom, which was based on the ‘one gene-one enzyme’ concept of Tatum and Beadle in the early 1940s [56].
The G Protein-Coupled Receptor (GPCR) represent the largest protein family encoded by the human genome and the most prominent gene target for therapeutic agents GCPRs are nested in the GCX of the cell membrane, which binds extracellular substances and transmits signals to an intracellular molecule called a G protein (guanine nucleotide-binding protein) [57,58]. GCX disruption affects the GPCRs downstream signaling pathways and the enzymes associated with these downstream pathways are the targets of drug development [59]. In the search for therapeutics vs. SARS-CoV, the inhibition of the GPCR enzyme RNA-dependent RNA polymerase is the target of two drugs under development: Remdesivir and monupril [60]. Remdesivir, a failed hepatitis C drug, which is repurposed to compete with Adenosine Triphosphate (ATP) [61,62].
Molnupiravir, a synthetic nucleoside derivative N4-hydroxycytidine, which is incorporated into the virus during viral RNA replication resulting in viral suicide or viral error catastrophe [63,64].
Vaccines target the early ‘downstream’ phase, which make them effective. A semblance of a vaccine is the Poly I:C, an immunomodulatory acting as a Toll-Like Receptor-3 (TLR3) agonist that triggers the production of type I interferon (IFN), Dendritic Cells (DCs) and Natural Killer (NK) T-cells [65-67]. Poly I:C is being repurposed vs. COVID-19 [68].
In this regard, Poly IC was chosen as the control compound to compare the ‘upstream’ targeting EMB. EMB, particularly compound H, reduced viral load by 20.9% compared to 11.8% for poly IC, indicating that the more upstream the target, the more effective the therapy. The proposed action of EMB includes repair of glycocalyx, antioxidant (ROS) and anti-inflammatory (Figure 9).
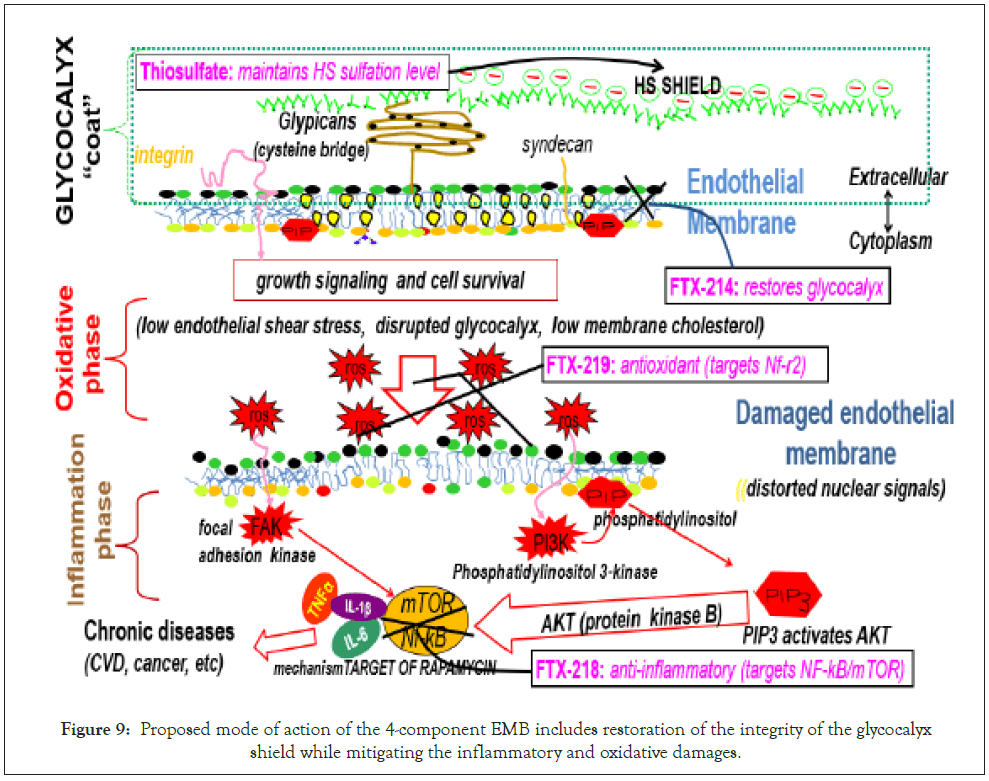
Figure 9: Proposed mode of action of the 4-component EMB includes restoration of the integrity of the glycocalyx shield while mitigating the inflammatory and oxidative damages.
On a molecular level, thiosulfate provides adequate sulfation to the heparan sulfate to maintain a negative shield and FTX-214 restores endothelial GCX integrity as a powerful anti-inflammatory and antioxidant agent [69,70].
In FTX-219 is an antioxidant that turns on the nuclear factor erythroid 2 related factor 2 (Nrf2), which is a major regulator of inducible defense systems against harmful endogenous and exogenous substances including viruses [71]. Viral infection suppresses Nrf2, consequently the hundreds of antioxidants and cytoprotective genes, which include Hemeoxygenase-1 (HO-1), Aldoketoreductase (AKR), Glutamine Cysteine Ligase (GCL), Glutathione Transferase (GST), Glutathione Synthetase (GS), Glutathione Peroxidase (GPx), NADH Quinine Oxidoreductase (NQO), Peroxiredoxin (Prx1), Superoxide Dismutase (SOD), Catalase (CAT), Thioredoxin Reductase (TrxR), Thioredoxin (Trx), UDP-Glucuronosyl Transferase (UGT) [72,73].
On the other hand, FTX-218 inactivates the Nuclear Factor Kappa b (NF-Kb), which is a transcription factor involved in inflammatory immune response [74,75]. Inflammatory is due to hyperactivation of Phosphoinositol 3 Kinase (PI3K)/protein kinase B (AKT)/ mammalian Target of Rapamycin (mTOR) (PI3K/AKT/mTOR) pathway particularly in viral infections [76].
Thus, the individual components synergize to restore in toto the health of the cell. Otherwise, lack of understanding on disease etiology leaves for hit-and-miss drug development paradigm. For example, repurposing drugs with individual attributes including blocking virus replication (faviravir, recombinant ACE2, lopinavir/ ritonavir); use of antibodies (bamlanivimab, convalescent plasma); anti-inflammatory agent (Rebif interferon, tocilizumab, colchicine, dexamethasone, baricitinib, azithromycin, stem cells), and anecdotal (Ivermectin, Oleandrin, Hydroxychloroquine/chloroquine, blood filtration, ACE inhibitors, vitamin, and mineral supplements) [77].
In summary, EMB effectively reduced the viral load of SARS CoV- 1, by synergistically targeting the upstream disease etiology and restore in toto the health of the cell to ward off diseases including viral infection.
The author would like to thank Brett Hurst and his staff for carrying out the viral reduction experiment Exp. PHA-249. The Institute for Antiviral Research (IAR). Utah State University, Logan Utah. 84322-5600 USA.
Citation: Tunac JB (2021) A Combo Compound Therapy Effectively Reduces SARS-CoV-1 Viral Load in Mouse Lung. J Antivir Antiretrovir. 13:221.
Received: 24-Mar-2021 Accepted: 07-Apr-2021 Published: 14-Apr-2021 , DOI: 10.37421/1948-5964.21.13.221
Copyright: © 2021 Tunac JB. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.