Short Communication - (2021)Volume 7, Issue 8
A Commentary on Bacterial Inoculation Positively Affects the Quality and Quantity of Flax under Deficit Irrigation Regimes
Sanaz Rajabi-Khamseh1*, Abdolrazagh Danesh Shahraki1, Mohammad Rafieiolhossaini1 and Kramatollah Saeidi2Abstract
The present research was conducted to investigate the effect of Plant Growth Promoting Rhizobacteria (PGPR) and deficit irrigation on quality and quantity of flax under field conditions. The effects of PGPRs were evaluated at different irrigation levels. Bacterial strains solubilized phosphate, produced ammonia, indole acetic acid, and siderophore. According to the results bacterial inoculations significantly mitigated the effects of water deficit on oil, linolenic and linoleic acid, protein, sulphur and vitamin contents, shoot and capsules number and harvest index.
Keywords
Bacterial inoculation; Deficit irrigation; Fatty acid; Flax; Oilseed; Protein
About the Study
Oilseed crops are mainly grown for the oil in their seed. Oil extracted from flax is used for industrial purposes. The quantity and quality of oilseed crops are affected by drought stress. There are a variety of soil microorganisms that safeguard plants against drought stress. PGPRs can promote plant productivity and health through of substances secretion, antifungal compounds production and plant systematic resistance induction [1]. The present study was planned and implemented to examine the effects of PGPR strains application on the quality and quantity of flaxseed under deficit irrigation regimes.
Factors comprised of irrigation (100% (NS), 75% (MS), and 50% (SS) crop water requirements) and bacterial treatments (control (C), Azotobacter chroococcum (A1), Azospirillum lipoferum (A2), Bacillus amyloliquefaciens (B1), Bacillus sp. strain1 (B2), and Pseudomonas putida (P)). Qualitative test of bacteria were included phosphate solubilization [2], ammonia [3], indole acetic acid (IAA)- like compounds [4], and siderophore [5,6] production. Deficit irrigation was based on maximum allowable water depletion [7]. The content of oil [8], protein [9], sulfur [10], and thiamin [11] and unsaturated fatty acids composition [12] as quality traits and the number of shoots and capsules and harvest index [13] as quantity traits were measured in plants maturation stage.
Analysis of the Study
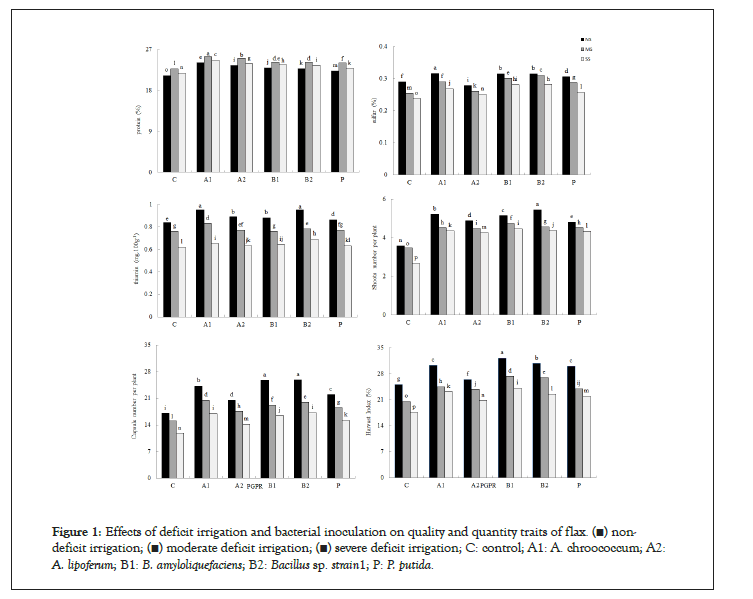
The plant growth-promoting features of the inoculum bacteria are shown in Table 1. Oil and unsaturated fatty acid contents decreased in water-deficit conditions, while bacterially inoculated treatments resulted in higher content, compared with those of the control plants (Table 2). Water deficit stress was observed to increase protein content in all plants. However, the bacterially inoculated plants showed higher content compared to those observed in C plants for all three irrigation regimes. Sulfur and thiamin content, shoot and capsules number, and harvest index were also higher in fully irrigated and bacterially inoculated plants (Figure 1).
Figure 1: Effects of deficit irrigation and bacterial inoculation on quality and quantity traits of flax. (▪) nondeficit irrigation; (▪) moderate deficit irrigation; (▪) severe deficit irrigation; C: control; A1: A. chroococcum; A2: A. lipoferum; B1: B. amyloliquefaciens; B2: Bacillus sp. strain1; P: P. putida.
| A1 | A2 | B1 | B2 | P | |
|---|---|---|---|---|---|
| Phosphate solubilization | H+ | L+ | H+ | M+ | H+ |
| Ammonia production | + | + | + | - | + |
| IAA-like compound production | + | + | + | + | + |
| Siderophore production | + | + | + | + | - |
Note: +: positive activity, –: no activity, H: high, M: medium and L; low phosphate solubilization ability.
Table 1: Plant growth promoting properties of inoculum bacteria.
| Traits | Stress | C | A1 | A2 | B1 | B2 | P |
|---|---|---|---|---|---|---|---|
| NS | 29.6j | 44.5a | 33.2g | 44.7a | 39.9b | 36.9d | |
| Oil | MS | 28.8k | 34.6f | 30.7i | 39.4c | 34.7f | 33.4g |
| SS | 25.1n | 31.8h | 26.4m | 36.2e | 30.5i | 26.8l | |
| NS | 50.64m | 55.89b | 52.79i | 55.8c | 55.70d | 56.34a | |
| Linolenic | MS | 50.14n | 55.72d | 51.5k | 54.74f | 54.32g | 55.51e |
| SS | 49.71o | 53.63h | 50.94l | 50.94l | 51.93j | 49.76o | |
| NS | 16a | 15.18b | 14.42g | 14.78f | 15.05c | 15.18b | |
| Linoleic | MS | 14.93d | 14.15jk | 14.3h | 14.71f | 14.39g | 14.24hi |
| SS | 14.88de | 14.11kl | 14.03m | 14.05lm | 14.21ij | 14.86e |
Note: NS: non stress; MS: moderate stress; SS: severe stress; C: control; A1: A. chroococcum; A2: A. lipoferum; B1: B. amyloliquefaciens; B2: Bacillus sp. strain1; P: P. putida; Means sharing same letter(s) were not significantly different.
Table 2: Effects of deficit irrigation and bacterial inoculation on oil and unsaturated fatty acid contents of flaxseed under field conditions.
Discussion
In the present research, inoculation with PGPRs improved the quality and quantity of flax plants under deficit irrigation, when compared with non-inoculated treatments. By converting insoluble phosphate to soluble forms, PGPRs enhance phosphate absorption by plants. By facilitating nitrogen availability (in the form of ammonia), PGPRs can play a significant role in providing other nutrients for yield production. IAA-producing bacteria may promote increased root surface area by improving root growth, which facilitates nutrient uptake from the soil. Siderophore compounds were secreted to solubilize iron from the surrounding environment of the plants and to form a ferric-siderophore complexes, which can be absorbed by plants [14]. Reduction in seed quality could be due to producing of small and medium sized seeds under water deficit conditions [15]. As indicated by the findings of the current study, the bacteria used in this research might increase oil content in inoculated plants through different mechanisms such as nitrogen availability, mineralization and solubilization of nutrients, IAA production, improved iron nutrition, and tolerance against water stress. By increasing absorption of nutrients, biomass production will increase and subsequently, the oil content will also increase due to production adequate photosynthetic products. It has been reported that photosynthetic function is associated with the relative proportion of unsaturated fatty acids unsaturation under unfavorable conditions. Especially abiotic stresses cause inactivation of photosystem I and II. It is thought that membrane ![]() lipid fatty acid unsaturation is protective to photosystems [16]. Therefore, growth-promoting bacteria may play a significant role in protecting photosynthetic systems by increasing unsaturated fatty acid, such as linolenic acid levels, under stress conditions. Therefore, in the current study the application of PGPRs, as biofertilizer, may have affected nutrient absorption, photosynthesis, and production of precursors which are essential in the synthesis of fatty acids. The elevated protein contents in inoculated plants is expected since protein is a nitrogen source and the inoculum bacteria (specially Azotobacter and Azosporillium strains) can produce ammonia and fix nitrogen for plant uptake. This metabolism can increase nitrogen availability in soil providing conditions for protein synthesis. Sulfur plays a vital role in oil biosynthesis in oilseed crops. In their experiment with tobacco plants, [17] found that in comparison to control plants, Bacillus sp.B55-inoculated plants exhibited increased plant growth which was attributed to increased sulfur content via sulfur volatile uptake. Vitamins which are essential for plant growth can be produced by PGPR [18]. The enhanced thiamine content observed in inoculated plants may be related to the bacterial growth-promoting properties (Table 1), especially IAA production which increases the availability of nutrients like sulfur (one of the elements present in thiamin structure). The increased number of shoots detected in the inoculated flax plants may be due to the alleviation of drought stress by the PGPR possible acting through phytohormones such as IAA which promotes root and shoots development. Aslantal et al. [19] attributed bacterial IAA and gibberellic acid production to increased numbers of shoots in inoculated young apple trees. Water deficit leads to stomatal closure and decrease in the water content of aerial organs that subsequently decreases the number of capsules. However, PGPRs can increase photosynthesis and capsule production by affecting root nutrient and water uptake. By delaying leaf aging and increasing leaf area duration, bacterial IAA may lead to increased net photosynthesis, and consequently increased production of capsules. Greater harvest index recorded in the inoculated plants might be related to the growth-promoting properties (Table 1) of the bacteria used in the study. Inoculation of chickpea plants with the combined form of PGPRs (Bacillus subtilis, B. thuringiensis, and B. megaterium) eportedly increased harvest index, in comparison with non-inoculated treatments [20].
lipid fatty acid unsaturation is protective to photosystems [16]. Therefore, growth-promoting bacteria may play a significant role in protecting photosynthetic systems by increasing unsaturated fatty acid, such as linolenic acid levels, under stress conditions. Therefore, in the current study the application of PGPRs, as biofertilizer, may have affected nutrient absorption, photosynthesis, and production of precursors which are essential in the synthesis of fatty acids. The elevated protein contents in inoculated plants is expected since protein is a nitrogen source and the inoculum bacteria (specially Azotobacter and Azosporillium strains) can produce ammonia and fix nitrogen for plant uptake. This metabolism can increase nitrogen availability in soil providing conditions for protein synthesis. Sulfur plays a vital role in oil biosynthesis in oilseed crops. In their experiment with tobacco plants, [17] found that in comparison to control plants, Bacillus sp.B55-inoculated plants exhibited increased plant growth which was attributed to increased sulfur content via sulfur volatile uptake. Vitamins which are essential for plant growth can be produced by PGPR [18]. The enhanced thiamine content observed in inoculated plants may be related to the bacterial growth-promoting properties (Table 1), especially IAA production which increases the availability of nutrients like sulfur (one of the elements present in thiamin structure). The increased number of shoots detected in the inoculated flax plants may be due to the alleviation of drought stress by the PGPR possible acting through phytohormones such as IAA which promotes root and shoots development. Aslantal et al. [19] attributed bacterial IAA and gibberellic acid production to increased numbers of shoots in inoculated young apple trees. Water deficit leads to stomatal closure and decrease in the water content of aerial organs that subsequently decreases the number of capsules. However, PGPRs can increase photosynthesis and capsule production by affecting root nutrient and water uptake. By delaying leaf aging and increasing leaf area duration, bacterial IAA may lead to increased net photosynthesis, and consequently increased production of capsules. Greater harvest index recorded in the inoculated plants might be related to the growth-promoting properties (Table 1) of the bacteria used in the study. Inoculation of chickpea plants with the combined form of PGPRs (Bacillus subtilis, B. thuringiensis, and B. megaterium) eportedly increased harvest index, in comparison with non-inoculated treatments [20].
Conclusion
The observed improvement in qualitative and quantitative traits may be attributed to the phosphate solubilization, ammonia, IAA- like compounds, and siderophore production of the used bacteria. Taken together application of PGPR for increasing agricultural products is gaining noticeable importance and seems to be the trend for future especially under water deficit conditions.
References
- Sansinenea E. Bacillusspp.: As plant growth-promoting bacteria. Secondary Metabolites of Plant Growth Promoting Rhizomicroorganisms. 2019:225-237.
- Baig KS, Arshad M, Zahir ZA, Cheema MA. Comparative efficacy of qualitative and quantitative methods for rock phosphate solubilization with phosphate solubilizing rhizobacteria. Plant Soil Environ. 2010; 29(1):82-86.
- Goswami D, Dhandhukia P, Patel P, Thakker JN. Screening of PGPR from saline desert of Kutch: growth promotion in Arachis hypogea by Bacillus licheniformis A2. Microbiol Res. 2014; 169(1):66-75.
- Glickmann E, Dessaux Y. A critical examination of the specificity of the Salkowski reagent for indolic compounds produced by phytopathogenic bacteria. Appl environ microbiol. 1995; 61(2):793-796.
- Hu QP, Xu JG. A simple double-layered chrome azurol S agar (SD-CASA) plate assay to optimize the production of siderophores by a potential biocontrol agent Bacillus. Afr J Microbiol Res.2011;5(25):4321-4327.
- Louden BC, Haarmann D, Lynne AM. Use of blue agar CAS assay for siderophore detection. J microbiol biol educ. 2011;12(1):51-53.
- Allen RG, Pereira LS, Raes D, Smith M. Crop evapotranspiration-Guidelines for computing crop water requirements-FAO irrigation and drainage paper 56. Fao, Rome. 1998; 300.
- AOCS O. Methods and recommended practices of the American Oil Chemists’ Society. American Oil Chemists' Society, Champaign, IL, USA. 1998; 5:2-93.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J boil chem. 1951; 193:265-275.
- Kalra Y. Handbook of reference methods for plant analysis. Soil and Plant Analysis.1997.
- Yousaf A, Qadir A, Anjum T, Ishaq Khan DR, Naughton D, Yousaf A. Evaluation of bacterial strains for the induction of plant biochemicals, nutritional contents and isozymes in barley. J Nutr Food Sci. 2017; 7(5):623.
- Kates M. Techniques of lipidology; isolation, analysis and identification of lipids. Lab tech in biochem mol biol. 1972; 3:347-353.
- Kozak M, MÃ?dry W. Note on yield component analysis. Cereal Res Commun. 2006; 34(2-3):933-940.
- Andrews SC, Robinson AK, Rodríguez-Quiñones F. Bacterial iron homeostasis. FEMS microbiology reviews. 2003; 27(2-3):215-237.
- Alqudah AM, Samarah NH, Mullen RE. Drought stress effect on crop pollination, seed set, yield and quality. InAlternative farming systems, biotechnology, drought stress and ecological fertilisation 2011; 193-213.
- Allakhverdiev SI, Kinoshita M, Inaba M, Suzuki I, Murata N. Unsaturated fatty acids in membrane lipids protect the photosynthetic machinery against salt-induced damage in Synechococcus. J Plant Physiol. 2001; 125(4):1842-1853
- Meldau DG, Meldau S, Hoang LH, Underberg S, Wünsche H, Baldwin IT. Dimethyl disulfide produced by the naturally associated bacterium Bacillus sp B55 promotes Nicotiana attenuata growth by enhancing sulfur nutrition. The Plant Cell. 2013; 25(7):2731-2747.
- Palacios OA, Bashan Y, de-Bashan LE. Proven and potential involvement of vitamins in interactions of plants with plant growth-promoting bacteria—an overview. Biol fertil soils.2014; 50(3):415-432.
- Aslantaà ? R, Cakmakçi R, à ?ahin F. Effect of plant growth promoting rhizobacteria on young apple tree growth and fruit yield under orchard conditions. Sci Hortic. 2007; 111(4):371-377.
- Khan N, Bano AM, Babar A. Impacts of plant growth promoters and plant growth regulators on rainfed agriculture. PloS one. 2020; 15(4):e0231426.
Author Info
Sanaz Rajabi-Khamseh1*, Abdolrazagh Danesh Shahraki1, Mohammad Rafieiolhossaini1 and Kramatollah Saeidi22Department of Horticulture, Faculty of Agriculture, Shahrekord University, Shahrekord, Iran
Citation: Khamseh SR, Shahraki AD, Rafieiolhossaini M, Saeidi K (2021) A Commentary on Bacterial Inoculation Positively Affects the Quality and Quantity of Flax under Deficit Irrigation Regimes. Appli Microbiol Open Access. 7: 210.
Received: 03-Aug-2021 Accepted: 17-Aug-2021 Published: 24-Aug-2021 , DOI: 10.35248/2471-9315.21.7.210
Copyright: © 2021 Khamseh SR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.