Clinical & Experimental Cardiology
Open Access
ISSN: 2155-9880
ISSN: 2155-9880
Research Article - (2017) Volume 8, Issue 3
Chicken meat is generally thought to be health friendly due to its favorable fatty acid composition leading to an antiatherogenic lipidemic status. Beneficial effect of the chicken oil on hyperglycemia has also been claimed through its effect on oxidative stress and insulin resistance. To further explore the potential benefits the effects of the oil from domestic and hybrid chicken for their fatty acid composition as well as for their effects on glycemic and lipidemic status in diabetic model rats were studied. There were significantly lower blood glucose value in DG and DDO groups at endpoint compared to baseline (mmol/l, mean ± SD, Baseline vs. Endpoint, 17.6 ± 0.98 vs. 16.5 ± 1.02; p= 0.001 and 18.4 ± 1.86 vs. 18.0 ± 1.0 ; p=0.001, for the DG and DHO groups at Endpoint compared to Baseline (mmol/l, mean ± SD, Baseline vs. Endpoint, 17.6 ± 0.98 vs. 16.5 ± 1.02; p= 0.001 and 18.9 ± 1.19 vs. 17.8 ± 1.84; p=0.001). for the DG and DDF groups at Endpoint compared to Baseline (mmol/l, mean ± SD, Baseline vs. Endpoint, 17.6 ± 0.98 vs. 16.5 ± 1.02; p= 0.001 and 19.2 ± 2.35 vs. 17.6 ± 2.19; p=0.001) and for the DG and DHF groups at Endpoint compared to Baseline (mmol/l, mean ± SD, Baseline vs. Endpoint, 17.6 ± 0.98 vs. 16.5 ± 1.02; p=0.001 and 19.9 ± 2.77 vs. 18.0 ±1.47; p=0.001). Chicken oil also showed hypolipidemic effects. 20% and 24% reduction of cholesterol level were observed for domestic and hybrid chickens oil treatment respectively in diabetic rats whereas 13% and 16% reduction were seen for domestic and hybrid chicken flesh. In case of glibenclemide, it was 33%. LDL level was reduced for domestic chicken oil, hybrid chicken oil, domestic chicken flesh, hybrid chicken flesh and glibenclemide at 19%, 20%, 16%, 18% and 22%. Whereas HDL level was increased significantly 33%, 37%, 26%, 32% and 44% respectively. The SGPT, SGOT and CRP were significantly decrease (p<0.05). Chickens oil has also effect of hepatoprotective activity.
Keywords: Broiler, Free range, Meat, Fatty acid, In Situ Transesterification
Lipids are important constituents of food and living cells. Dietary lipids play important roles in the energy production process of animal tissues as a source of essential fatty acids (EFA). Essential fatty acids are polyunsaturated fatty acids, among which n-3 fatty acids have potential health benefits [1]. Other polyunsaturated fatty acids and monounsaturated fatty acids are also associated with many health benefits like coronary heart disease. According to American Heart Association (AHA), regular dietary intake of n-3 fatty acids have some protective health effects such as, decrease in platelet aggregation, reduction in triglyceride levels, retardation of atherosclerosis and anti-inflammatory effects [2]. As recommended by the WHO people should take in fat which should represent less than 30% of the daily energy needed, but there can be no more than 10% of saturated fatty acids (SFA) and 3% -7% of polyunsaturated fatty acids (PUFA). From these linoleic and -linolenic acids are essential for people [3]. Animal feed enriched by nutritionally important various fatty acids can improve the nutritive value of animal fat. Adding linseed oil rich in the -linolenic acid results in a significant influence of this fatty acid on its amount in chicken lipids. Adding herring oil to chicken feed increases the shares of eicosapentaenoic (EPA), docosapentaenoic (DPA) and docosahexaenoic (DHA) acids [4]. Essential fatty acids are closely associated with the risk of diabetes. It was reported that Type 2 Diabetes is strongly associated with proinflammatory products in obese tissue and it has been established that insulin resistance results from inflammation of the adipose tissue in which cytokines such as tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, and (IL)-6 as well as products such as PAI-1 (plasminogen activator inhibitor-1) are part of the development and progression of T2D. In this regard, omega-3 fatty acids should be beneficial and prevent T2D because they have the potential of suppressing the production of these proinflammatory products in macrophages and adipocytes of adipose tissue [5]. Fatty acid composition is also depends on nature of food with its environment. Grass fed chickens, reared outdoors has a more favorable fatty acid composition than those of the extensive indoor rearing chickens. The share of essential fatty acids as well as polyunsaturated omega-3 and omega-6 fatty acids is greater amount in outdoor rearing chickens than indoor rearing ones and they have favorable fatty acid composition, which probably should also be reflected in the more favorable lipid composition of such chickens. This poultry meat is an important provider of the essential polyunsaturated fatty acids (PUFAs). Domestic chickens are particularly good source of EFA because of their diet. The general objective of the study is the comparative studies on fatty acid composition of domestic and hybrid chickens and their effect on blood parameters in experimental diabetic rats.
Materials and Method
Sample collection and preparation
Collection of chickens: Both domestic and hybrid chicken, ten of each type were collected from the nearby market at Jahangirnagar University area. The collected chickens were cleaned, air-dried, packed in polyethylene bag, sealed and stored at 4°C for use in subsequent experiments. Only the meat portion of chicken was used for various experimental purposes.
Animals (Rats) care: Test animals were collected from International Cholera and Dysentery Disease Research, in Bangladesh (icddr, b). Albino rats (wistar strain) of both sexes weighing 175 g (average) were used for the study and also recruited both gender. They were individually housed in polypropylene cages in well-ventilated rooms, under hygienic conditions. Feeding of animals was done a libitum, along with drinking water and maintained at natural day night cycle.
Extraction of oil: Oil was extracted from hybrid and domestic chicken material with n-hexane by Soxhlet apparatus according to [6]. The extract was evaporated under reduced pressure in a rotary evaporator to obtain the oil.
GC Mass Analysis of Fatty Acids in domestic and hybrid chickens oil
Fatty acid composition of extracted oil was determined by Gasliquid chromatography according to [7]. Gas chromatography was conducted with a Gas Chromatograph GC-2025 series with AOC-20i Auto Injector (Shimadzu Co, Japan). GC- 2025 Gas chromatography status include temperature -280ºC, pressure-175.4 KPa, total flow-165 ml/min, purge flow 3 ml/min, Column temperature -270ºC. Domestic and hybrid chickens’ oil was first saponified to produce the free fatty acid salts. The fatty acid salts then are derivatized to form the fatty acid methyl esters (FAME) according to the American Oil Chemists Society (AOCS). The FAME was extracted with a non-polar solvent (e.g., hexane) for analysis by GC.
Each FAME (Fatty Acid Methyl Ester) in extract was identified by comparing retention times with those of known standard FAME (Lipid Standard Sigma chemical Co, St Louis, MO, USA). The area of fatty acids was measured with GC solution 2011. The results were expressed as relative percentage of fatty acids. The relative percentage of fatty acids was calculated by the formula:
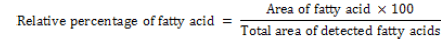
Domestic and hybrid chicken oil on fasting blood glucose level
Induction of diabetics: Diabetes was induced in overnight fasted rats by a single intraperitoneal injection of 5% solution of Alloxan monohydrate (110 mg/kg body weight) (Sigma Chemical Co., USA) in a 0.1 M sodium citrate buffer (pH-4.5). The age-matched control rats received an equivalent amount of citrate buffer. Food and water intake were closely monitored daily after Alloxan administration. The development of hyperglycaemia in rats was confirmed by fasting (16 h) blood glucose measurement in the tail vein blood, 48 hours after Alloxan administration, with a Portable glucometer (Accu-Chek, Roche, Germany). The animals with fasting blood glucose level ≥ 11.0 mmol/L with other symptoms of diabetes mellitus such as polyphagia, polydipsia, polyuria, and weight loss were considered diabetic and included in the study.
Blood collection: Blood samples from all groups were collected on days 7, 14 and 21 in a fasting state from rat’s marginal ear vein by 26 G needle and syringe [8]. Blood glucose level was determined by “Humylazer 2000” analyser (Human, Germany). The values was expressed as mean ± S.E.M, Statistical analyses were performed by SPSS-16 one-way analysis of variance (ANOVA), followed by posthoc Tukey’s test for multiple comparisons. P<0.05, P<0.001 were considered as significant.
Experimental animals grouping and treatment: The animals were randomly divided into four groups. Each group contain six rats (n=6). The treatment of animals began on the initial day after Alloxan injection and this was considered as 1st day of treatment. The animals were treated for 3 weeks as follows:
Group 1: control rats feed with standard pellet diet and water.
Group 2: The rats were made diabetic by an intra-peritoneal injection of single dose of 110 mg/kg body weight followed by of 5% solution of Alloxan monohydrate. Animals whose blood glucose level exceeded 11.0 mmol/L at 72 h after treatment were considered diabetic. These animals served as untreated diabetic control.
Group 3: The diabetic rats treated with Diabetic+domestic chicken oil (1% of total diet), Diabetic+hybrid chicken oil (1% of total diet) extract solution dose for 21 days.
Group 4: Diabetic rats were treated by Glibenclamide at a dose of 0.5 mg/kg b.wt.
Group 5: Diabetic+domestic chicken flesh (1% of total diet).
Group 6: Diabetic+hybrid chicken flesh (1% of total diet).
Measurement of blood parameters
Plasma concentrations of triglyceride (TG), total cholesterol (TC), HDL-cholesterol (HDL-C), LDL-cholesterol (LDL-C), VLDL, serum creatinine, urea, uric acid, SGPT, SGOT and CRP were measured using a quantification kits (Linear chemicals, Barcelona, Spain) by automatic Bioanalyzer (Hitachi 7180, Hitachi, Tokyo, Japan).
Fatty acid composition of Domestic and Hybrid Chicken oil was illustrated in (Table 1). Both the oils contain myristic acid behenic acid in a very small amount. These two represent a minor saturated constituent of the oil. Bulk of the saturated fatty acids is presented as C16:0 or palmitic acid and C18:0 or stearic acid, however C16:0 or palmitic acid was the major saturated fatty acid of the total fatty acids. The monoenoic acids oleic acid (C18:1) had the highest proportion of the total oil. Palmitoleic acid (C16:1) was the next monoenoic acid in the hybrid chicken oil but domestic chicken contain no palmitoleic acid. Both, domestic and hybrid chicken oil contains arachidonic acid in a small quantity. Domestic chicken contains relatively higher amount of arachidonic acid.
| Name of fatty acid | Domestic chicken (Rel%) | Hybrid chicken (Rel%) |
|---|---|---|
| Myristic Acid | 0.24 | 0.34 |
| Palmitic Acid | 19.62 | 17.55 |
| Oleic Acid | 75.75 | 48.4 |
| Stearic Acid | 1.84 | 4.67 |
| Arachidonic Acid | 1.67 | 0.49 |
| Behenic Acid | 0.85 | 0.2 |
| Palmotelic Acid | - | 28.31 |
| Others | 0.03 | 0.04 |
Table 1: Fatty acid composition of Domestic and Hybrid Chicken oil.
Effect of Domestic and Hybrid chicken oil and flesh are reported in (Table 2). After first week of chicken oil treatment the domestic and hybrid chicken oil and chicken flesh, had no significant effect on blood glucose level of rat, But after 3rd week of treatment there was a significant hypoglycemic effect had seen, comparing the blood glucose level in alloxan induced rats. The blood glucose levels were decreased by 12% and 10% for Domestic and hybrid chicken oil administered subject whereas 8% and 9% for Domestic and hybrid chicken flesh administered subject which is as near as glibenclamide administered subject (16%).
| Treatment Group | 7 Days | 14 Days | 21 Days |
|---|---|---|---|
| NDC | 5.1 ± 1.59 | 5.3 ± 1.63 | 5.2 ± 1.21 |
| DC | 19.12 ± 1.8 | 21.2 ± 1.09 | 21.9 ± 1.99 |
| DG | 17.5 ± 0.98 | 17.21 ± 1.03 | 16.4 ± 1.02 |
| DDO | 18.5 ± 1.86 | 18.2 ± 1.12 | 18.0 ± 0.99* |
| DHO | 18.9 ± 1.19 | 18.5 ± 1.91 | 17.8 ± 1.84* |
| DDF | 19.2 ± 2.35 | 19.0 ± 1.83 | 17.6 ± 2.19* |
| DHF | 19.9 ± 2.77 | 19.9. ± 1. | 18.0 ± 1.47* |
| *Indicate significance change compared with normal control group (P<0.05). The results are expressed as ± SEM.Group: NCD - normal ( non-diabetic rats ), DC- control (Alloxan induced diabetic rat without treatment), DG- diabetic+glibenclemide, DDO- Diabetic+domestic chicken oil (1% of total diet), DHO- Diabetic+hybrid chicken oil (1% of total diet), DDF- Diabetic + domestic chicken flesh(1% of total diet), DHF- Diabetic+hybrid chicken flesh (1% of total diet). | |||
Table 2: Comparative effect of Domestic and Hybrid Chicken oil on fasting blood glucose level of experimental rats, 21 days study,(n=6).
(Table 3) showed the results of body weight control rats were increased after 2nd week. Initially diabetic rats decrease their body weight but after 2nd week of chicken oil chicken flesh treatment, there was an increase in body weight. Domestic and hybrid chicken oil Increase of body weight by 20% and 17% respectively. On the other hand Domestic and hybrid chicken flesh feeding rats, increase body weight around 18% and 22%, which areas near as the glibenclamide treatment group, it was 21%.
| Treatment Group | 7 Days | 14 Days | 21 Days |
|---|---|---|---|
| NDC | 92.58 ± 1.24 | 130.37 ± 0.64 | 122.83 ± 0.37 |
| DC | 103.88 ± 1.94 | 88.69 ± 0.65 | 74.61 ± 0.52 |
| DG | 94.54 ± 0.54 | 109.12 ± 0.46 | 114.94 ± 0.63* |
| DDO | 94.49 ± 2,98 | 105.3 ± 2.58 | 113.52 ± 2.64* |
| DHO | 95.72 ± 3.10 | 104.07 ± O.588 | 111.05 ± 4.59* |
| DDF | 92.27 ± 2.43 | 101.63 ± 3.17 | 111.38 ± 1.75* |
| DHF | 94.16 ± 1.63 | 98.42 ± 1.35 | 113.56 ± 2.54* |
| *Indicate significance change compared with normal control group (P<0.05). The results are expressed as ± SEM. Group: NCD - normal ( non-diabetic rats ), DC- control (Alloxan induced diabetic rat without treatment), DG- diabetic+glibenclemide, DDO- Diabetic+domestic chicken oil(1% of total diet), DHO- Diabetic+hybrid chicken oil(1% of total diet), DDF- Diabetic+domestic chicken flesh(1% of total diet), DHF- Diabetic+hybrid chicken flesh (1% of total diet). |
|||
Table 3: Comparative effect of Domestic and Hybrid Chicken oil on the body weight of experimental rats, 21 days study (n= 6).
The results of the lipid profile of non-diabetic and diabetic rats are shown in (Table 4) 20% and 24% reduction of cholesterol level were observed for domestic and hybrid chickens oil treatment respectively in diabetic rats whereas 13% and 16% reduction were seen for domestic and hybrid chicken flesh. In case of glibenclemide, it was 33%. LDL level was reduced (P<0.001) for domestic chicken oil, hybrid chicken oil, domestic chicken flesh, hybrid chicken flesh and glibenclemide at 19%, 20%, 16%, 18% and 22%. Whereas HDL level was increased significantly 33%, 37%, 26%, 32% and 44% respectively. A little bit different scenario was observed for triglyceride level. Triglyceride reduced the triglyceride level more significantly than chicken oil and flesh. Triglyceride level reduced for domestic chicken oil, hybrid chicken oil, and domestic chicken flesh, hybrid chicken flesh by around 9%, 11%, 8% and 6% respectively. Whereas Triglyceride level was reduced by 39%. Table 4 represented the change of lipid profile by the administration of chicken oil and flesh in alloxan induced diabetic rats.
| Treatment Group | 21 Days study | |||
|---|---|---|---|---|
| Cholesterol | Triglyceride | HDL | LDL | |
| NDC | 70.44 ± 0.68 | 72.07 ± 0.27 | 50.83 ± 0.50 | 85.9 ± 0.513 |
| DC | 97.97 ± 0.46 | 110.22 ± 0.36 | 45.085 ± 0.15 | 121.10 ± 0.314 |
| DG | 64.82 ± 0.33 | 67.05 ± 0.17 | 64.98 ± 0.16 | 94.04 ± 0.215 |
| DDO | 78.045 ± 0.18 | 99.98 ± 0.18 | 59.98 ± 0.17 | 97.99 ± 0.137 |
| DHO | 74.08 ± 0.25* | 97.07 ± 0.17 | 62.165 ± 0.28* | 96.08 ± 0.220* |
| DDF | 85.41 ± 1.15 | 102.35 ± 1.58 | 57.11 ± 1.57* | 97.99 ± 1.76 |
| DHF | 80.33 ± 0.74* | 103.21 ± 2.43 | 60.31 ± 1.82* | 92.08 ± 1.17* |
| *Indicate significance change compared with normal control group (P<0.05). The results are expressed as ± SEM.Group: NCD - normal ( non-diabetic rats ), DC- control (Alloxan induced diabetic rat without treatment), DG- diabetic+glibenclemide, DDO- Diabetic+domestic chicken oil (1% of total diet), DHO- Diabetic+hybrid chicken oil (1% of total diet), DDF- Diabetic+domestic chicken flesh(1% of total diet), DHF- Diabetic+hybrid chicken flesh (1% of total diet). | ||||
Table 4: Comparative effect of Domestic and Hybrid Chicken oil on blood lipid profile of rats, 21days study, (n= 6).
Effect of chicken oil and flesh were shown in (Table 5). Results showed that after induction of diabetic, serum uric acid, urea and creatinine level were found to be high in diabetic rats but a little decrease was found after treatment with chicken oil and flesh. Uric acid level were 25%, 31%, 15%, 16% and 37% for domestic chicken oil, hybrid chicken oil, domestic chicken flesh and hybrid chicken flesh and glibenclamide treated group respectively compared with control group. Serum urea levels were reduced by 19%, 23%, 14%, 17% and 38% for domestic chicken oil, hybrid chicken oil, domestic chicken flesh and hybrid chicken flesh and glibenclamide treated group respectively compared with control group. Creatinine levels reduction were observed by 21%, 33%, 17%, 19% and 43% for domestic chicken oil, hybrid chicken oil, domestic chicken flesh and hybrid chicken flesh and glibenclamide treated group respectively compared with control group.
| Treatment Group | Uric acid (mg/dl) | Urea (mg/dl) | Creatinine |
|---|---|---|---|
| (mg/dl) | |||
| NDC | 7.871 ± 0.28 | 32.41 ± 0.43 | 1.24 ± 0.17 |
| DC | 16.00 ± 0.29 | 56.47 ± 0.45 | 2.72 ± 0.16 |
| DG | 10.03 ± 0.44 | 35.47 ± 0.45 | 1.54 ± 0.14 |
| DDO | 12.05 ± 0.32 | 45.98 ± o.42 | 2.11 ± 0.15 |
| DHO | 10.89 ± 0.37* | 43.08 ± 0.26* | 1.79 ± 0.31* |
| DDF | 14.05 ± 1.26 | 48. 08 ± 1.36 | 2.53 ± 1.33 |
| DHF | 13.89 ± 1.07* | 46.98 ± 1.15 | 2.19 ± 1.03 |
| *Indicate significance change compared with normal control group (P<0.05). The results are expressed as ± SEM.Group: NCD - normal ( non-diabetic rats ), DC- control (Alloxan induced diabetic rat without treatment), DG- diabetic+glibenclemide, DDO- Diabetic+domestic chicken oil (1% of total diet), DHO- Diabetic+hybrid chicken oil (1% of total diet), DDF- Diabetic+domestic chicken flesh(1% of total diet), DHF- Diabetic+hybrid chicken flesh (1% of total diet). | |||
Table 5: Comparative effect of domestic and hybrid chicken oils on serum creatinine, urea, uric acid level of experimental rats, 21-days study, n=6.
(Figure 1) represent the effects of chicken oil and chicken flesh on serum SGPT and SGOT level of alloxen induced diabetic rats. SGPT levels were found to be reduced by 21%, 30%, 20%, 21% and 34% for domestic chicken oil, hybrid chicken oil, domestic chicken flesh and hybrid chicken flesh and glibenclamide treated group respectively compared with control group. Similar effect had seen for SGOT level. The level was decreased by 11%, 14%, 9%, 12%, 16% and 19% for domestic chicken oil, hybrid chicken oil, domestic chicken flesh and hybrid chicken flesh and glibenclamide treated group respectively compared with control group.
(Figure 2) showed the CRP (C - reactive protein) level of domestic and hybrid chicken oil of alloxen induced diabetic rats. C - Reactive protein (CRP) level was higher than the non-diabetic group. By the treatment of domestic and hybrid chicken oil and flesh CRP significantly reduces 25.06% from 61.32% diabetic group whereas glibenclamide reduce 42.0% from 61.32%. By the treatment of oil and flesh significantly reduction of CRP level 23.51% from 67.14% in diabetic group (p<0.05).
As lipid serves as an efficient source of energy and dietary lipids are involved in most of our body processes but not all lipids are good for health [9]. A study also reported Substitution of dietary saturated fat by oleic acid and polyunsaturated fatty acids (PUFA) has been described to reduce the cardiovascular risk by reducing blood lipids, mainly cholesterol. Additional benefits have been described for long chain omega-3 PUFA-eicosapentaenoic acid-EPA and docosahexaenoic acid-DHA Oleic acid is a common monounsaturated fat in human diet. Monounsaturated fat consumption has been associated with decreased low-density lipoprotein (LDL) cholesterol, and possibly increased high-density lipoprotein (HDL) cholesterol [10]. However, its ability to raise HDL is still debated [11]. Oleic acid may be responsible for the hypotensive (blood pressure reducing) effects of olive oil. Adverse effects also have been documented, however, since both oleic and monounsaturated fatty acid levels in the membranes of red blood cells have been associated with increased risk of breast cancer, although the consumption of oleate in olive oil has been associated with a decreased risk of breast cancer [12]. Arachidonic acid is a polyunsaturated omega-6 fatty acid 20:4 (ω-6). It is the counterpart to the saturated arachidic acid. Arachidonic acid is not one of the essential fatty acids. However, it does become essential if there is a deficiency in linoleic acid or if there is an inability to convert linoleic acid to arachidonic acid, which is required by most mammals. Some mammals lack the ability to or have a very limited capacity to convert linoleic acid into arachidonic acid, making it an essential part of their diets [13]. Obesity is a healthcare problem today and its prevalence has increased greatly in recent decades in almost every country and all age group. The major cause in the recent obesity epidemic is a changing environment that promotes excessive calorie intake and discourages physical activity, causing an energy imbalance. According to WHOs latest projections approximately 1.6 billion adults (age 15+) were overweight worldwide in 2015 and at least 400 million adults were obese. WHO further estimates that by 2015, approximately 2.3 billion adults will be overweight and more than 700 million will be obese [14]. After induction of diabetes alloxan induced diabetic rats became thin and their weight was reduced. For normal rats, their weights were increased substantially after 2nd and 3rd week. Initially diabetic rats decrease their body weight but after 2nd week of chicken oil chicken flesh treatment, there was an increase in body weight. The result showed that hybrid chicken lowers the blood glucose level of rats and maintains it at standard level more significantly than domestic chicken. The plasma glucose lowering activity was compared with glibenclamide, a standard hypoglycemic drug. Glibenclamide has been used for many years to treat diabetes, to stimulate insulin secretion from pancreatic β cells [15]. 20% and 24% reduction of cholesterol level were observed for domestic and hybrid chickens oil treatment respectively in diabetic rats whereas 13% and 16% reduction were seen for domestic and hybrid chicken flesh. In case of glibenclemide, it was 33%. LDL level was reduced for domestic chicken oil, hybrid chicken oil, domestic chicken flesh, hybrid chicken flesh and glibenclemide at 19%, 20%,16%,18% and 22%. Chicken consumption also exerts a potential effect in reducing cholesterol, triglyceride, LDL level and increase HDL level. In this case, hybrid chicken plays more important role than domestic chicken [16]. SGPT and SGOT is typically used to detect liver injury. It is often ordered in conjunction with SGOT. These two tests are considered as a most important test to detect injury. SGPT and SGOT are known to be associated to the liver and the red blood cells. The destruction of the liver and red blood cells triggers the release of these enzymes into circulation [17]. The level was decreased by 11%, 14%, 9%, 12%, 16% and 19% for domestic chicken oil, hybrid chicken oil, domestic chicken flesh and hybrid chicken flesh and glibenclamede treated group respectively compared with control group. Wheather it was oil or flesh, hybrid chicken oil and flesh reduced serum SGPT and SGOT level more significant way [18]. C -reactive protein (CRP) is a simple cost effective test, which can predict the cardiovascular risk. The addition of CRP- testing to standard lipid screening appears to provide an important method to determine Cardiovascular Disease (CVD) risk factor [19,20]. Dietary supplementation of HFO declined CRP level significantly and thus reduced the risk of cardiovascular diseases.
The study aimed to investigate the meat composition of both type of chickens mentioned above. From the study, it may be concluded that, Fatty acid composition of the oil showed that domestic chicken contains oleic acid and arachidonic acid in greater amount, which have very beneficial health effect. On the other hand, presence of a large amount of plamoteleic acid is noticed only in hybrid chicken. It is a ω-7 monounsaturated fatty acid which has also cholesterol, TG and LDL lowering property. Comparing the blood sugar level in alloxan induced rats, domestic chicken oil and hybrid chicken oil administered subject shows significant reduction of blood sugar. Domestic and Hybrid chicken oil Increase body weight by 38% and 40% respectively which was as near as the glibenclemide treatment reduction of cholesterol level were observed for domestic and hybrid chicken oil treatment respectively. There was a massive increase in the SGOT, SGPT and CRP level and also a little increase in SGOT level after diabetes induction which was compensated by domestic chicken oil and hybrid oil significantly.
The Authors declare that they have no competing interests.