Medicinal & Aromatic Plants
Open Access
ISSN: 2167-0412
ISSN: 2167-0412
Research Letter - (2015) Volume 4, Issue 3
Diabetes mellitus is metabolic syndrome that causes disability, early death, and many other complications like neuropathy, nephropathy, retinopathy, amputation, erectile dysfunction and cardiovascular disease. The study was designed to evaluate anti-diabetic activity and anti-toxicity of aqueous and methanolic extracts of M. communis. The extracts were chemically screened for phytochemicals. Anti-diabetic effect of aqueous and methanolic extracts of M. communis were separately evaluated by administering these extracts orally to alloxan induced diabetic mice at single dose of 500, 750 and 1000 mg/kg body weight. On the other hand, the antitoxicity studies of the aqueous extract of M. communis indicated that the extract is non-toxic and safe.The results indicate that mean lethal dose (LD50) for aqueous extract of M. communis was 8125 mg/kg. Aqueous extract of M. communis significantly lowered mean blood glucose level of diabetic mice at dose of 500 mg/kg by 61.8% (p<0.003) on the 5th hr. In contrast the methanolic extract of M. communis also significantly lowered mean blood glucose level by 48% (P<0.00003) at 1000 mg/kg dose level. These findings of our study were in agreement with previous reports. Further investigations are recommended to identify active blood glucose lowering chemicals in these plant extracts and elucidation of their mechanism of action.
Keywords: Diabetes mellitus; M. communis; Aqueous extract; Methanolic extract; Blood glucose level; Phytochemicals; Alloxan; Mice
Diabetes mellitus is a serious global health problem characterized by hyperglycemia which is caused by absolute or relative deficiency of insulin or by insulin resistance at the cellular level [1]. The disease is related with reduced quality of life and increased risk factors for mortality and morbidity [2]. Excessive food consumption regarding to high calorie, obesity, cardiovascular disease, stress, and lack of exercise are risk factors for diabetes mellitus [3]. According to International Diabetes Federation currently around 40.9 millions of peoples are diabetic and number is expected to rise to 69.9 million by 2025 unless urgent preventive steps are taken [4].
Different types of oral hypoglycemic agents are available along with insulin for the management of diabetes mellitus [5]. Although new and more efficacious diabetes medications and improved medication delivery systems have been developed, the majority of diabetic patients do not achieve optimal blood glucose control, leading to poor health outcomes and needing the news alternative drugs for treatment. It is the fact that diabetes cannot be cured and it has never been reported that someone had recovered totally from diabetes [6]. However, herbal treatment is an alternative in the treatment of this pathophysiology in as much as plants are used to cure diabetes [7]. Thus searching for a new class of compounds is essential to overcome diabetic problems [8]. Currently several hundred plants have been reported to have beneficial effect in treatment of diabetes [9].
M. commuins known ‘Ades’ in Amharic is an evergreen shrub which has been grown in the Mediterranean regions for centuries. It can reach up to 4.5 m tall, but smaller when regularly pruned [10]. A striking feature of the plant is the pleasant smell of its essential oil, present in numerous glands, especially in the leaves effects of the myrtle leaves were reported [11]. M. communis is one of medicinal plants used in Ethiopia. The leaves, fruits and volatile oil of M. communis are frequently used as folk remedies for several diseases [12]. In traditional medicine, the leaves and fruit of Myrtus communis are used as antiseptic, antibacterial [13], analgesic, in lung and digestive disorders and in the treatment of many types of infectious including candidacies, anti-inflammatory agent, a mouthwash antioxidant, antimicrobial, and anti-hyperglycemic [14,15]. A wide range of biologically active compounds such as tannins, flavonoids, coumarins, essential oil, fixed oil, fibres, sugars, citric acid, malic acid and antioxidants are present in the plant [16]. Therefore, this study was designed to evaluate antidiabetic activity and acte toxicity of aqueous and methanolic extracts M. communis.
Plant material
Fresh leaves of M. communis were collected from Bale Zone, Goba town, near the River Togona (7°0’N; 39°9’E), 2743 meters above sea level. Authentication and taxonomic identification of the plant samples was done by using standard botanical monographs at Addis Ababa University science faculty herbarium. The sample specimens of our study plants were identified as M. communis at Addis Ababa University science faculty herbarium. A portion of the sample was kept in the department museum for further reference.
Extraction
Fresh leaves M. communis were cleaned with distilled water and air dried at room temperature. Each of these medicinal plants was separately ground into powder using grinding mill at EHNRI Department of Drug Research. The powdered plant material was stored in glass jar. The aqueous extract was prepared by the maceration with double distilled water. Methonolic extract was made by maceration for 24 hrs. concentrating under reduced pressure using rotary evaporator at 50 rpm and was placed on water bath at 40°C to evaporate methanol completely.
Inducing experimental diabetes
Diabetic condition was induced to Swiss albino mice of both sexes, weighing 25-35 g after overnight fasting by intra peritoneal (IP) injection of freshly prepared alloxan (200 mg/kg, IP) dissolved in distilled water [17]. Three days (72 hrs) after the injection of alloxan, the animals were fasted for 16 hrs, and their blood-glucose concentration was determined with glucometer. Animals showing stabilized diabetes (FBG ≥ 250 mg/dL) on the third day after alloxan injection were selected for the experiment [18] (Jaouad and Badia, 2002). Mice that do not show diabetic condition on the third day were excluded from the experiment.
Laboratory animals
Healthy Swiss albino mice weighing 25 g-35 g were obtained from Ethiopian Health and Nutrition Research Institute (EHNRI) animal unit and kept in laboratory animal house of Drug Research Department during the experiment. The animals were provided standard pellet food and tap water ad libitum with 12 hr. light/dark photoperiod. The experiment was conducted according to the ethical norms approved by Animal Ethics Committee Guidelines of the EHNRI.
Experimental design
Alloxan induced experimental diabetic Swiss albino mice (FGB ≥ 250 mg/dL) were divided in to 9 test groups with 5 mice in each group that match with weight and fasting blood glucose level [19]. Body mass of each diabetic mouse in a group was measured and amount of extract for each test group was calculated, weighed, dissolved in distilled water and administered to each mouse PO. Group 1-3: Diabetic mice treated with crude aqueous extract of M. communis a dose of 500 mg/kg, 750 mg/kg, and 1000 mg/kg PO. Group 4-6: Diabetic mice treated with methanolic extract of M. communis at dose of 500 mg/kg, 750 mg/kg, and 1000 mg/kg PO. Group 7: Diabetic mice treated standard drug Glibenclamide 10 mg/kg PO [20]. Group 8: Untreated diabetic mice (Diabetic control) given equal volume of distilled water PO. Group 9: Non-diabetic reference group (Normal Control) given equal volume of distilled water P.O. Blood glucose level of these mice was measured using glucometer at interval of 1 hr after treatment for consecutive 5 hrs. by drawing blood from tail vein.
Acute toxicity test in mice
The mice were divided into 10 groups with 6 animals in each group matched for weight and sex. These animals were housed 3 mice per sex per cage in a well-ventilated room with 12 hrs. cycle of day and night light conditions and temperature maintained at around 25°C. Food was withdrawn for the 12 hrs fasting period before and for 24 hrs fasting period after oral administration of the single doses crude aqueous extract [18,21]. Aqueous extract of either M. communis was aseptically suspended in distilled water and administered at a single doses of 0, 1000, 2000, 3000, 4000 , 5000, 7500, 10000 and 12000 mg/ kg body weight by gavages per-oral (PO) to the test groups. The control groups were given an equal volume of water. The change in general behaviors of the mice was continuously monitored [22]. The number of dead mice was recorded and used in the calculation of mean lethal dose (LD50) value. All surviving animals were euthanized with diethyl-ether at day 14 as described by Ogwal-Okeng et al. [23].
Phytochemical screening
Aqueous extracts of M. communis leaves were tested qualitatively for presence of major secondary metabolites. The qualitative test was done according to procedure described by Debella [24]. The method was based on observation of color formation that occurs due to reaction of secondary metabolites with different standard reagents.
Stastical analysis
All the results of blood glucose were expressed as mean ± standard deviation for (n=5) animals in each group. The significance of difference between paired data was analyzed by student’s t-test. The results with p-values of less than 0.05 were considered statistically significant. The graphs were plotted using Microsoft Office Excel 2007. The results from diabetic groups treated with aqueous and methanolic extracts of M. communis were compared with the result of untreated diabetic mice (diabetic control).
Oral acute toxicity of M. comunis aqueous extracts
The oral acute toxicity study of the more active of the two extracts, which is the aqueous extract, was done to confirm the safety of the extracts at various doses. There was a regular dose-dependent increase in mortality of mice after the PO administration of M.communis (Table 1). Signs of toxicity also became more severe at higher doses. Total of 14 mice (7 male and 7 female) died out of 18 mice received aqueous extract of M.communis in dose range of 7500-12000 mg/ kg. Substances with LD50 between 500 mg/kg body weight and 5000 mg/kg of body weight are regarded as being slightly toxic while those with LD50 between 5000 mg/kg of body weight and 15000 mg/kg of bodyweight regarded as practically non-toxic [25]. Therefore oral dose of M. communis aqueous extract is practically non toxic and safe for use, since its LD50 is greater than 5000 mg/kg.
The aqueous extract of M. communis not caused death of experimental mice up to dose of 5000 mg/kg body weight. The median lethal dose (LD50) of aqueous M. communis calculated from Table 1 was 8125 mmg/kg. Therefore oral dose of M. communis aqueous extract is practically non toxic through oral route since its LD50 is greater than 5000 mg/kg. LD50 of the tested extracts were calculated according to the formula below [26]:
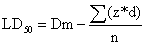
| Dose of M. communisaqueous extractin mg/kg | Number ofmice per group (n=6) | Number ofmice dead and Percentdead | Signs of toxicity | |
|---|---|---|---|---|
| 0.00 mg/kg | Group-1 | M=3 | 0/3 and 0% | None |
| F=3 | 0/3 | |||
| 1000 mg/kg | Group-2 | M=3 | 0/3 and 0% | None |
| F=3 | 0/3 | |||
| 2000 mg/kg | Group-3 | M=3 | 0/3 and 0% | None |
| F=3 | 0/3 | |||
| 3000 mg/kg | Group-4 | M=3 | 0/3 and 0% | None |
| F=3 | 0/3 | |||
| 4000 mg/kg | Group-5 | M=3 | 0/3 and 0% | None |
| F=3 | 0/3 | |||
| 5000 mg/kg | Group-6 | M=3 | 0/3 and 0% | Hypo activity, piloerection |
| F=3 | 0/3 | |||
| 7500 mg/kg | Group-7 | M=3 | 1/3 and 33.3% | Hypo activity, piloerection |
| F=3 | 1/3 | |||
| 10000 mg/kg | Group-8 | M=3 | 3/3 and 100% | Convulsion, hyperventilation, hypo activity, salivation |
| F=3 | 3/3 | |||
| 12000mg/kg | Group-9 | M=3 | 3/3 and 100% | Convulsion, hyperventilation, hypo activity, salivation |
| F=3 | 3/3 | |||
Table 1: Acute toxicity of M. communis aqueous extract in Swiss albino mice (n=6).
Where:
Dm=The largest dose which kill all animals.
z=Mean of dead animals between 2 successive groups.
d=The difference between 2 successive doses.
n=Number of animals in each group.
The result of calculated LD50 for aqueous extract of M. communis was 8125 mg/kg.
Hypoglycemic effect of aqueous and methanolic extracts of M. communis
In present study we have evaluated anti-diabetic activities of aqueous and methanolic extract of M. communis by using alloxan induced diabetic mice. Aqueous extract of M. communis leaves significantly lowered blood glucose level in diabetic mice at dose of 500 mg/kg (p<0.001). This single dose given via oral rout has lowered blood glucose level of diabetic mice by 61.8% (p<0.001) on 5th hr after treatment (Table 2). The methanol extract of M. communis also significantly lowered blood glucose (48% from 313 to 162 mg/dL, p<0.001) at doses of 1000 mg/kg in the 5th hr after treatment. On the other hand higher doses fail to significantly lower blood glucose of diabetic mice. This could be due to desensitization of receptors. In contrast to our finding Aylin and his coworkers reported significant blood glucose lowering effect of M. communis at dose of 50 mg/kg.
| Treatment | Mean change of blood glucose (mg/dL) in hrs after treatment (n=5) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0hr | 1hr | 2hr | 3hr | 4hr | 5hr | %Reduce | |||
| Diabetic control | 322±6.3 | 385.5±6.8 | 392.2±5.9 | 382.25±6.1 | 384.7±6.2 | 377.2±6.4 | 0.03% | ||
| AQ. Myrt 500 | 376±2.2 | 228.3±2.1 | 250±2.5 | 180.3±2.3* | 152±1.8* | 143.3±2.0* | 61.8% | ||
| AQ.Myrt 750 | 319.2±0.81 | 319±0.72 | 282±0.93 | 281.5±0.85 | 251±0.83 | 242±0.79 | 24.4% | ||
| AQ.Myrt1000 | 356.7±0.45 | 349.7±0.51 | 359.7±0.49 | 366.7±0.52 | 358.5±0.58 | 343.75±0.6 | 3.6% | ||
| Myrt Alco 500 | 303.5±5.8 | 290.2±5.3 | 283.75±5.5 | 274±6.7 | 268.75±7 | 259.75±6.9 | (14%) | ||
| MyrtAlco 750 | 351±7.3 | 268.7±7.1 | 272.75±7.4 | 238.7±7.6 | 233±8.2 | 231.5±7.8 | (34%) | ||
| MyrtAlco 1000 | 313±3.2 | 240±2.8 | 210±2.9 | 186±2.6** | 180±2.2** | 162±2.4** | (48%) | ||
| Normal control | 110.5±0.22 | 112±0.21 | 114±0.23 | 105±0.28 | 95.25±0.31 | 101±0.25 | 8% | ||
*p<0.01 as compared to diabetic control ** p<0.001 as compared to diabetic control
Table 2: Hypoglycemic effect of M. communis aqueous and methanolic extract in alloxan induced diabetic mice (n=5).
Comparing the hypoglycemic effects of aqueous and methanolic extracts of M. communis, the aqueous extract has higher activity at relatively lower dose (500 mg/kg) than the methanolic extract at 1000 mg/kg dose (Figure 1). This finding manifests the presence of the active ingredients in the aqueous extract at higher concentration than the in methanolic extract. This is due to the fact that the most active portions are manifested in higher polarity.
Phytochemical in aqueous extract of M. communis leaves
Previous studies reported presence of Polyphinoles, Flavonoids, Tannins and other phytochemical in M. communis [27,28]. Finding of phytochemical screening in our study agrees with these previous works except for absence of flavonoids in M. communis used in our study (Table 3).
| Secondary metabolite | Reagents and method | Indicators | M. communis |
|---|---|---|---|
| Chromophers | 10ml distilled water+heat for 30min | Yellow to red color | +++ |
| Polyphenols | 1%FeCl3+1ml of K3 [Fe (CN)6] | Green blue color | +++ |
| Saponins | 30ml distilled water +heat(5min) | Formation ofhoney comb froth | +++ |
| Phytosteroides and withanoids | CHCl3+conc. H2SO4 | Red, reddish brown or violet color | _ _ _ |
| Flavonoids | 5 drops of 2% lead acetate | Yellow or orange color | _ _ _ |
| Tannins | 3drops of 1%K3[Fe(CN)6]+ 3drops of conc. NH3 | Formation of color | +++ |
| Antraquinone glycosides | 2N HCL, benzene, 10% ammonia | Red color | _ _ _ |
Table 3: Results of preliminary phytochemical analysis in aqueous crude extracts of M. communis leaves.
As shown in acute toxicity test of this study, the aqueous extract of M. communis were found to be non-toxic through oral rout, since their LD50 is greater than 5000 mg/kg. As it was elucidated by Sepici et al. [21] M. communis extract stimulate insulin secretion in normoglycemic rabbits. However insulin secretion was not significant and also didn’t work for alloxan diabetic rabbits. Therefore it is difficult to suggest about its mechanism of action at this level of our study.
Aqueous and methanolic extracts of the medicinal plant have significantly lowered blood glucose level in alloxan induced diabetic mice. However, the aqueous extract has higher activity at relatively lower dose (500 mg/kg) than the methanolic extract at 1000 mg/ kg dose. This indicates the aqueous extract has a better efficacy in contrast to the methanolic extract as anti-diabetic agent. Therefore intensive investigations must be conducted on these extracts to identify pharmacologically active compound(s), to elucidate mechanism of action, to assure their safety and set appropriate dose for human diabetic patients. The herb reduces blood glucose and may have beneficial effects in treating complications of diabetes.