Journal of Agricultural Science and Food Research
Open Access
ISSN: 2593-9173
ISSN: 2593-9173
Research - (2019)Volume 10, Issue 3
Millets are important food crops in semi-arid and tropical regions of Africa and Asia. The cooking and eating quality of foods are determined by the rheological, functional and color properties of flours. In this research, four finger millet varieties (Axum, Padet, Tadese and Tesema) and one pearl millet variety (Kola-1) were collected. The aim of this study was to evaluate the rheological, functional and color characteristics of improved millet varieties grown in Ethiopia. A significant (p<0.05) variations were observed among the five millet flours in their pasting profiles. Pearl millet variety, Kola-1 showed the highest flour solubility (12.86%), pasting temperature (77.4°C) and the lowest flour swelling power (195.54 g/g), peak viscosity, trough viscosity, breakdown viscosity, setback viscosity, final viscosity and falling number with a values of 855 cP, 293.5 cP, 561.5 cP, 540 cP, 833.5 cP and 197.5 sec, respectively compared to finger millet varieties. Padet had the highest flour water absorption index (3.24 g/g), pasting profiles (3434 cP, 1704 cP, 1730 cP, 2042 cP and 3746 cP) and the lowest pasting temperature (75.02°C). The water absorption capacity of flours was ranged from 116.11 to 120.68%, the highest for flours from Tesema. Kola-1 was significantly differed in gel length and gel consistency from other varieties and was found to have a short gel length and hard gel. In terms of color properties, Kola-1 had the highest L* (57.11) and âE (41.85) values for grain, b* (4.21) value for flour, b* (8.4) and âE (42.14) values for injera. Baking resulted in a significant reduction in L* value and increment in a* and b* color values.
Millet; Injera; Pasting property; Gel consistency; Hunter color
Cereals are staple foods for a large proportion of the world population. Millets are one of the economically important cereals in the world asides the major cereals wheat, rice, maize and oat. These crops are important in semi-arid and tropical regions of Asia and Africa due to their resistance to diseases, pests, short growing season and ability to thrive in less fertile soils under heat and drought conditions [1,2]. There are four major types of millets, namely pearl millet (Pennisetum glaucum), which comprises 40% of the world production, foxtail millet (Setaria italica) [3], proso millet or white millet (Panicum miliaceum), and finger Millet (Eleusine coracana) and with a total production of 762,712 tonnes [2,3] and the top producer was India with an annual production of 334500 tons (43.85%) (FAO, 2012). Nutritionally, millets are equivalent to other cereal grains [2].
Millet is utilized in the form of injera, porridge, bread, and local beverage like tella and areki, etc. Injera is fermented, sour bread consumed as a staple food in Ethiopia and other neighboring countries. The bread can be prepared from various cereals but teff [Eragrostis tef (Zucc.) Trotter] is the most preferred ingredient. The food property is characterized by the structure, quality, nutritional value and /or acceptability of a food product. Nutrient compositions of cereals depend not only on genotype [4,5] but also on environmental factors [6]. Soil factors and soil-water relationships; weather and climatic factors; postharvest handling and storage; and fertilizer applications and cultural practices [7] and method of processing applied are some of a factors influencing grain composition and properties. Goswami et al. analyzed a number of pearl millet varieties of African, American and Indian origin and observed that variations in protein, fat, total ash, calcium, phosphorus and iron were large. This implies that genetic and environmental factors play a major role in determining grain composition and properties [8].
Rheological and functional properties are a fundamental physicochemical properties that reflect the complex interaction between the composition, structure, molecular conformation and physicochemical properties of food components together with the nature of environment in which these are associated [9-11]. The physicfunctional and rheological characteristics of flour determines the product quality and quantity. Meaning, these properties are central for evaluating the quality of both raw materials and end products and predicting its behavior during processing. Possibly it helps to predict mainly how starches, proteins, and fat behaves in specific systems [9,11]. In addition, rheological properties are important to the design of flow processes, recipe optimization, storage and processing stability measurements, predicting texture, and understanding the molecular and conformational changes in food materials [12]. Chakraborty et al. studied rheological properties of millet based dough under thermo-mechanical stress and successfully optimized the level of ingredients for wheat flour-millet based bread [13]. The amount and degree of amylose leaching rheological/pasting behaviour of starch, granule size distribution, volume fraction, shape, rigidity and extent of granule swelling, inter-granular interactions and continuous phase viscosity [14,15]. To our knowledge, very limited or no studies have been published for their rheological and functional behaviors on those improved millet varieties grown in Ethiopia. The aim of this study was to investigate the rheological, functional and color properties of millet grains, flours and injera.
In these study five samples of released millet varieties (Four finger millet and one pearl millet) namely Padet, Tessema, Tadesse, Aksum and Kola-1 grown in 2018/2019 season were collected from Melkassa Agricultural Research Center, Ethiopia. The millet grains were sorted, cleaned, washed, drained, sun dried and ground into flour. The experiment was conducted in food technology laboratory of Can Tho University, Vietnam and grain quality laboratory of Addis Ababa Science and Technology University, Ethiopia.
Rheological and functional properties
Pasting properties: Pasting properties of flours were studied by using Rapid Visco Analyzer (RVA) as described by Maninder et al. [16]. Viscosity profiles of flours were recorded using flours suspensions (8%; 28 g total weight). 3.5g of the flour sample was weighed into a dried empty canister; 25 ml of distilled water was dispensed into the canister containing the sample. The mixture was thoroughly stirred, and the canister was fitted into the RVA as per manufacturer’s instructions. A programmed heating and cooling cycle from 50°C to 95°C was used, where the suspension was held at 50°C for 1 min, heated at a uniform rate to 95°C for 8 min and then held at 95°C for 5 min before cooling to 50°C within 8 min, and finally held at 50°C for 1 min. The pasting profiles such as peak viscosity (PV), viscosity at trough (TV), and final viscosity (FV) were recorded, and breakdown (BDV, which is PV minus TV) and setback (SB, which is FV minus TV) were determined with the aid of Thermocline for Windows Software connected to a computer. Falling number (FN) was measured by Farinograph (Perten instruments, model FN 100, Sweden) as described in the application manual. 25 ml of deionized water was added to 7 g flour and mixed thoroughly. The total time in seconds from the immersion of the viscometer tube into the water bath until the viscometer stirrer has fallen the prescribed distance through the gelatinized suspension was taken.
Flour functional property: The swelling power and solubility of flours were determined as described by with a slight modification [17]. Flour samples of 1 g were mixed with 10 ml of distilled water in centrifuge tubes. The resulting suspensions were heated at a constant temperature (from 65°C to 95°C) in a water bath for 30 min. The gelatinized samples were cooled to room temperature and centrifuged at 3,000×g for 20 min. The supernatant was dried presumably in an oven at 105°C to a constant weight to quantify the soluble fraction. The solubility was expressed as the percentage of dried solid weight based on the weight of the dry sample. The swelling power was represented as the ratio of the weight of the wet sediment to the weight of the initial dry sample. Water absorption index was calculated based on the weight gain by the gel. The water absorption capacity of the flours was determine by the method of Sosulski et al. [18]. One gram of sample mixed with 10 ml distilled water and allow to stand at room temperature for 30 min, the centrifuged for 30 min at 2000×g. Water absorption was examined as per cent water bound per gram flour.
Swelling power (g/g) = Weight of the wet sediments/Weight of the dry flour (g)
Solubility (%) = (Weight of the dried supernatant/Initial weight of dry flour) × 100
WAI (g/g )=Weight gain by gel/Weight of dry flour
WAC (%) = Amount of water bound/Weight of dry flour
Gel consistency measurement: Gel consistency was measured using method [19]. Milled samples were finely grounded to a smallest particle size using mortar and pestle for three hours. By considering the initial moisture content of samples, 0.99 g triplicate samples were weighed in a test tube and 0.2 ml thymol blue and 2 ml 0.2 N KOH were added to each samples and thoroughly mixed. Then, the samples were put in a water bath at 100°C for five minutes with continuous stirring. Finally, the samples were cooled in a refrigerator for 20 minutes and laid horizontally on a table for 1 hr. A distance the gel flowed was recorded and classified as very soft (80-100 mm), soft (61-80 mm), medium gel (41-60 mm), flaky (35-40 mm) and very flakey (<35 mm). The degree of hardness of the gel was determined using the formula:
Gel consistency = 130 – gel length (mm)
Injera making
Injera preparation was conducted using standardized injera making procedure [20]. The procedure involved milling whole millet grain into a flour, preparation of dough, and fermentation of the dough for 48 hrs. About 500 g of the fermented batter was poured in a circular manner on a 50-cm diameter hot clay griddle, covered, and baked for 2 min. the baked injera was oven dried at 40°C and ground into injera flour.
Color characteristics
Color measurements of grain, flour and injera samples were carried out using a Hunter colorimeter (Model, NR60CP 3NH Technology Co., LTD) optical Sensor based on L*, a*, and b* values as described by Kaur and Singh [21]. A glass cell containing flour was placed above the light source, covered with a white plate and L*, a*, and b* colour values were recorded. The instrument (45o/0o geometry, 10 observer) was calibrated against a standard red-coloured reference tile (Ls = 25.54, as = 28.89, bs = 12.03). Total colour difference (ΔE) was calculated by applying the equation:
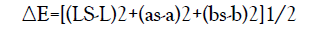
Where, the L* value indicates the lightness, 0–100 representing dark to light. The a* value gives the degree of the red-green colour, with a higher positive a* value indicating redder. The b* value indicates the degree of the yellow-blue colour, with a higher positive b* value indicating more yellow.
Data analysis
Data was analyzed using a one way analysis of variance (ANOVA) using Minitab 16 statistical software package and Tukey’s multiple comparison tests was used to determine the significance of differences among treatments at 95% confidence level. Each value was determined by at least duplicates. Results were given as mean ± standard deviation
Rheological and functional properties
Pasting properties: The behavior of starch in water is temperature and concentration dependent [22]. Pasting properties reflect the changes in viscosity of flour during heating in excess water under constant stirring conditions. The results of pasting properties of millet flours are indicated in Table 1. A significant (p<0.05) difference was observed between all millet varieties in their pasting temperature (PT). The lowest and highest PT was noticed in Padet (75.02°C) and Kola-1 (77.4°C) varieties. All finger millet cultivars were showed significant variation in their cooking temperature. The ability of starch to imbibe water and swell is primarily dependent on the pasting temperature with Padet to have the lowermost PT. Starch granules swell and form paste by imbibing water in the presence of water and heat [23]. The pasting temperature provides an indication of the minimum temperature required for cooking. Pasting properties indicate the tendency to form paste, the higher the pasting temperature, the faster the tendency for paste to be formed. The peak viscosity (PV) of millet cultivars was varied significantly and ranged from 855 (Kola-1) to 3434 cP (Padet). Padet and Tesema were found to have a statistically similar PV value. Similarly, Axum and Tadese were found to have a statistically similar PV value. During heating viscosity increased due to the swelling of granules to several times their original size, and due to the loss of crystalline order and absorption of water. PV indicates the water binding capacity and the maximum swelling of the starch granule prior to disintegration [24]. However et al. were stated that granules with high peak viscosity have weaker cohesive forces within the granules than those with lower values and would disintegrate more easily [25]. The amylose-to-amylopectin ratio of starch greatly affects the starch pasting properties. Falade and his co-workers showed that the peak viscosity and other physicochemical parameters including amylose, amylopectin and granule sizes of the starches of the cocoyam cultivars varied significantly [26]. In addition, pasting properties are greatly influenced by plant source, starch content, interaction among the components, and testing condition [24].
| Variety | PT (°C) | PV | TV | BDV | SBV | FV | Pti (min) | FN (sec) |
|---|---|---|---|---|---|---|---|---|
| Axum | 76.72 ± 0.03b | 2850 ± 43.80c | 1589.5 ± 81.3a | 1260.5 ± 37.5b | 1682.5 ± 68.6b | 2772 ± 857a | 5.70 ± 0.04a | 242 ± 0.00a |
| Kola-1 | 77.40 ± 0.00a | 855 ± 7.10d | 293.5 ± 6.4b | 561.5 ± 0.7c | 540 ± 38.2c | 833.5 ± 44.5b | 5.53 ± 1.13a | 197.5 ± 6.36b |
| Padet | 75.02 ± 0.03d | 3434 ± 18.4a | 1704 ± 56.6a | 1730 ± 38.2a | 2042 ± 79.2a | 3746 ± 22.6a | 5.63 ± 0.05a | 242 ± 0.00a |
| Tadese | 76.62 ± 0.03b | 2914.5 ± 125.2bc | 1646 ± 198a | 1268.5 ± 72.8b | 1852.5 ± 7.8ab | 3500 ± 203.6a | 5.76 ± 0.05a | 242 ± 0.00a |
| Tesema | 75.9 ± 0.00c | 3151 ± 86.3ab | 1640.5 ± 55.9a | 1510.5 ± 142.1ab | 1902 ± 100.4ab | 3542.5 ± 44.5a | 5.80 ± 0.09a | 242 ± 0.00a |
| Means in the same column followed by different alphabets are significantly different at α-5% level of probability by Tukey's Multiple Comparison .Test within and between different millet cultivars, n=2. PT, pasting temperature; PV, TV, BDV, FV, SBV are peak, trough, breakdown, final and setback viscosities respectively; Pti, peak time; FN, falling number | ||||||||
Table 1: Pasting profiles (cP) and falling number of millet flours.
The trough viscosity (TV) of the flours ranged from 293.5 to 1704 cP and Padet had the highest TV with non- significant (p>0.05) within finger millet cultivars (Axum, Padet, Tadese and Tesema). Pearl millet variety, Kola-1 attained the lowest TV, indicates the resistance of swollen granules towards shear. After reaching maximum, the viscosity decreased due to the rupturing and fragmentation of granules by stirring known as breakdown viscosity [27,28]. Breakdown viscosity (BDV) of millet varieties varied significantly between 561.5 and 1730 cP. Kola-1 had the lowest BDV (561.7 cP), which showed greater resistance to heat and shear. Padet (1730 cP) and Tesema (1510.5 cP) were found to have higher BDV than that of Axum and Tadese. BDV is a measure of paste resistance to disintegration in response to heat and shear, lower breakdown viscosity showed greater resistance which would be expected of flours with lower peak viscosities. Axum and Tadese showed the highest stability ratio (0.56) (ratio of trough to peak viscosities), which indicated most stable to shear; whereas Kola-1 had the lowest stability ratio (0.34), revealed least stable. This could be attributed to the increment of moisture content and as it increased the stability ratio. Stability ratio describes the resistance of starch paste to viscosity breakdown as shear is applied. Starches with a higher stability ratio have potential applications in heat processed products like soups. The setback values differed significantly (p<0.05) and ranged from 540 to 2042 cP, the highest for Padet and the lowest for Kola-1. The flours from Padet, Tadese and Tesema showed similar SBV value with non-statistical difference among them. SBV is an indication of how starch molecules behave after heating, cooking and cooling and determines the tendency of starch to retrogradation. The higher the setback value, the lower the retrogradation during cooling and the lower the staling rate of the products made from the flour. This might be due to a high amount of amylose as it’s more susceptible to retrogradation than amylopectin. Thus, Kola-1 could be more susceptible retrogradation with a retrogradation rate (ratio of setback to peak viscosities) of 0.63 than finger millet varieties. The final viscosity (FV) of Padet (3746 cP) was higher when compared to all flours with insignificant variation within finger millet cultivars while Kola-1 (833.5 cP) showed significant variation. FV indicates the ability of the flours to form a viscous paste or gel after cooking and cooling. A remarkable increase in FV was observed and this marked increase could be due to the alignment of the chains of amylose in the starch [29].
This study agreed to Gull, et al. reported the PV, breakdown viscosity, final viscosity, setback and pasting temperature of millets (Finger & pearl) flours were ranged from 429 to 3362 cP, 39 to 732 cP, 983 to 3902 cP, 593 to 1272 cP and 89.60°C to 74.35°C, respectively [30]. According to this study, pearl millet flour showed lower value for peak viscosity, breakdown viscosity, final viscosity and setback value, and higher pasting temperature compared to finger millet flour. Gomez, et al. stated that pastes with lower peak viscosity, breakdown, final viscosity and total set back is due to their minimal carbohydrate content and also their different protein content affecting the viscometric parameters [31,32]. Finger millet flour showed a high range of setback value (1272 cP) compared to pearl millet flour which showed only (593 cP) [32]. Rageaee et al. reported that the pasting temperature, peak time, peak viscosity, trough viscosity, final viscosity, breakdown viscosity and setback viscosity of millet blends (85% wheat flour + 15% whole grain millet) 86.9°C, 9.1 min, 1363 cP, 588 cP, 1826 cP, 775 cP and 1238 cP, respectively [33]. Peak time of the of millet flour samples ranged from 5.53 to 5.8 min, the longest for Tesema and the shortest for Kola-1 with non-significant (p<0.05) difference among all flours. Peak time was negatively correlated (r=-0.31) with pasting temperature and positively correlated (r=0.73) with peak viscosity. This indicated as high peak time characterized high swelling starch granules in the flour due to the presence of relatively high amylose content. Contrarily, PT was negatively correlated (r=-0.81) with PV.
Falling number (FN), which indicates α-amylase activity in sprout damaged grain, is defined as the total time in seconds from immersion of the viscometer tube into the water bath, until the viscometer stirrer fallen the prescribed distance through the gelatinized suspension. Kola-1 was differed significantly (p<0.05) and had the shortest time (197.5 seconds) which showed a relatively high amylase activity. However, no significant difference was observed in FN between finger millet varieties (Tadese, Tesema, Padet, and Axum). According to a manual of FN, a FN below 150 possess high amylase activity, meaning sprout damaged grain and between 200-300 possess optimal amylase activity; thus the result indicated low α-amylase activity in all finger millet flours when compared to pearl millet. Particle size and variety could affect the value of FN. Thus, Pearl millet was shown to have very high amylase activity, about ten times higher than that of wheat grain, and this was probably responsible for the low peak viscosity observed [34].
Amylase of pearl millet was observed to be more active against wheat starch than against the starch from pearl millet grain itself [35,36]. This observation has a great practical importance in food processing. Bread prepared from wheat flour blended with 10 percent pearl millet flour had better loaf volume than standard bread prepared from wheat flour containing malt and sugar [37]. Thus, pearl millet flour used in partial replacement of wheat flour can be successfully substituted for malt and sugar in the preparation of bakery products such as bread, biscuits and pasta.
Functional properties: The results of water absorption capacity (WAC), swelling power (SP), solubility and water absorption index (WAI) are indicated in Table 2. A significant difference was observed between and within the flours of millet varieties in their functional properties. Tesema had the highest (120%) WAC and was significantly different from other millet varieties. The lowest percentage of WAC was observed in Kola-1 (116.26%), statistically no significant difference with Axum, Padet and Tadese millet varieties. Several authors had been reported the WAC of pearl millet flour. Siroha, et al. reported that water absorption capacity of the pearl millet flours ranged from 153 to 177% [38]. Yadav and Oshodi, et al. reported WAC of 183% and 115%, respectively for pearl millet flour [39,40]. This variation could be due to different protein concentration, their degree of interaction with water and conformational characteristics, amylose solubility and the degree of opaqueness of the endosperm [41]. In food processing applications, functional properties determines how flours behave during preparation and cooking, and how it affects the finished food product in terms of appearance, tastes, and feels. High WAC of composite flours suggests that the flours can be used in formulation of some foods such as sausage, dough, and bakery products. The increase in the WAC has always been associated with increase in the amylose leaching and solubility, and loss of starch crystalline structure.
| Variety | Flour functional properties | |||
|---|---|---|---|---|
| WAC, % | Swelling power, g/g | Solubility, % | WAI, g/g | |
| Axum | 117.32 ± 0.70b | 170.04 ± 8.04c | 2.84 ± 0.099c | 2.70 ± 0.08c |
| Kola-1 | 116.26 ± 1.03b | 195.54 ± 0.76b | 12.86 ± 1.22a | 2.95 ± 0.01b |
| Padet | 117.07 ± 0.11b | 223.88 ± 0.17a | 4.08 ± 0.169bc | 3.24 ± 0.00a |
| Tadese | 116.11 ± 1.02b | 203.49 ± 3.55b | 5.79 ± 0.291b | 3.03 ± 0.04b |
| Tesema | 120.68 ± 0.44a | 205.93 ± 1.32b | 4.65 ± 0.355bc | 3.06 ± 0.01b |
| a, b, c, and d superscripts are significantly (p<0.05) different column wise within and between different millet cultivars, n=2; WAC, water absorption capacity; SP, swelling power; WAI, water absorption index | ||||
Table 2: Functional properties of millet flours.
A swelling power of flours ranged from 170.04 to 223.8 g/g. Among millet varieties Axum and Padet varieties had the lowest (170 g/g) and the highest (223.88 g/g) values respectively with a significant difference in between. On the other hand, Kola-1, Tadese and Tesema were statistically similar. Swelling power of flour describes the water holding capacity upon heating in water, followed by cooling, and centrifuging. A positive correlation (r= 0.31) was noticed between peak viscosity and swelling power. Higher SP of flour from Padet was probably due to its lower content of fat, grain hardness and longer chains in amylopectin structure. SP of starch has been reported to depend on the water holding capacity of starch molecules by hydrogen bonding [42]. SP is contributed by amylopectin content, Tester et al. and also the distribution across the granule, amylose/amylopectin distribution [43,44]. Rani, et al. indicated that starch granules with higher amylose content, being less rigid, swell freely when heated [45]. Conversely, starch granules with higher amylose being better reinforced and thus more rigid, probably swell less freely. The molecular structure of amylose and amylopectin, physical associations of chemical components in the granules, size distribution of granules, and presence of lipid– amylose complex could affect SP [25].
The highest and lowest percent solubility was observed in Kola- 1 (12.86%) and Axum (2.84%). In addition, Axum was differed from Tadese statistically (p<0.05) but not from Padet and Tesema. The result is comparable with Lorenz, et al. revealed the solubility of proso and foxtail millet starch at 60°C, 70°C, 80°C and 90°C ranged from 2.33 to 14.52 and 2.57 to 11.12, and 2.33 to 13.59% and 0.55 to 5.39% respectively [46]. Sandhu, et al. reported the SP and solubility of starches from pearl millet cultivars were in a range of 14.1 to 17.9 g/g and 10.4 to 16.2 g/100 g, respectively [47]. Solubility links to water loving and amylose (more the amylose leaching and hence solubility) content and it is the dissociation of inter and intra hydrogen bonds [48]. Swelling power and solubility provide measures of the magnitude of interaction between starch chains within the amorphous and crystalline domains [49]. The degree of this interaction is affected by the amylose to amylopectin ratio, the characteristics of amylose and amylopectin in terms of molecular weight/distribution, and the branching degree and chain length of amylose and amylopectin [50]. Starch granules absorb water and swell to several times their initial size, and components of starch granules, mainly amylose, leach out [51,52]. Subramanian, et al. observed that the quality of unleavened bread, roti prepared from pearl millet flours was influenced by swelling capacity, watersoluble flour fraction, water-soluble protein and amylose content of the flour [53]. According to this study, the swelling capacity of the flour was highly and positively correlated with all the sensory dualities of roti, namely color, texture, odor, taste and acceptability. On the other hand, the amylose content and water-soluble flour fraction were negatively correlated with all these characteristics. Beleia, et al. noticed that inherent molecular dissimilarity was the primary factor in physico-chemical differences among five pearl millet starches examined [54]. In addition, this study revealed that variation in the water-binding capacity 83.6 to 99.5% was probably due to differences in the ratio of amorphous and crystalline starch in the granule; amorphous starch has greater water absorption capacity than crystalline starch. Water absorption index (WAI), the ability of the flour to associate with water, was differed among millet varieties. A range of 2.7 to 3.24 g/g WAI was recorded, the lowest for Axum and the highest for Padet with statistical difference among them. However, Tesema, Tadese and Kola-1 statistically similar. Higher WAI is particularly useful in products where hydration is required to enhance handling characteristics, such as dough’s and pastes [55]. A positive but not significant correlation (r=0.18; p>0.05) was noticed between swelling power and percent solubility and between WAC, WAI and SP (r=0.52; p>0.05).
Gel consistency: Nutritional, appearance, cooking and eating quality are a characteristics of grain quality. Eating quality is determined mainly by the texture of a food (cohesiveness, tenderness, hardness). Gel consistency determines the cooking and eating quality of flours. More the distance (in mm) traveled by the starch gel, lower is the gel consistency. A strong and negative correlation (r=-1) was observed between gel length and gel consistency (Figure 1). Kola-1 showed significantly (p≤0.05) higher gel consistency (100 mm) and Tesema exhibited a lower gel consistency (76 mm). Tadese and Padet did not show a significant difference (p>0.05) in their gel consistency behavior.
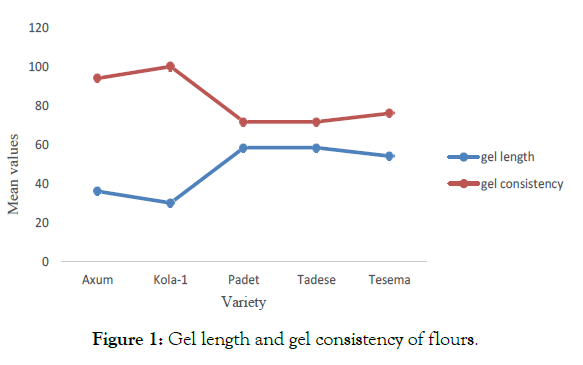
Figure 1: Gel length and gel consistency of flours.
Hence, Kola-1 and Axum were classified as very flaky (30-35 mm) flour and had the greater tendency to harden on cooling than other finger millet varieties (Figure 2). Tesema showed a characteristic of medium gel with a value of 54 mm (p<0.05) than Tesema and Padet. The difference in gel consistency within finger millet cultivars might be due to their amylose and protein contents. Unnikrishnan, et al. noticed that gel consistency is proportional to the sediment volume of aqueous starch dispersion at room temperature [56]. Tan, et al. noted that the amylose content, gel consistency and gelling temperature are generally considered to be the three most important traits that determine the cooking and eating quality of rice [57].
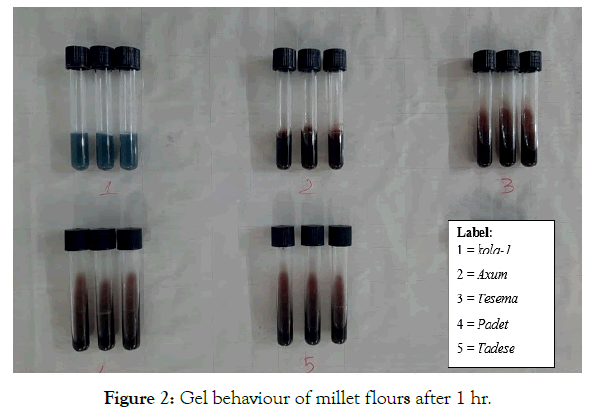
Figure 2: Gel behaviour of millet flours after 1 hr.
Color characteristics: Color parameters (L*, a*, b*and ΔE) of grains, flours, and fermented and then baked injera were evaluated using hunter color lab and the results are shown in Tables 3 and 4. The L*, a*, b* and ΔE values of the grains ranged from 44.34 to 57.11; 1.68 to 10.44; 3.77 to 9.53 and 29.30 to 41.85, respectively. Kola-1 had the highest L* value and the lowest a* value than Tesema and other varieties with a significant difference between them.
| Variety | Color characteristics | Injera | ||||||
|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | ∆E | L* | a* | b* | ∆E | |
| Axum | 62.01 ± 0.237c | 1.14 ± 0.178a | 2.81 ± 0.217b | 46.75 ± 0.18c | 49.69 ± 0.27b | 3.84 ± 0.11b | 4.83 ± 0.15c | 35.53 ± 0.23bc |
| Kola-1 | 59.76 ± 0.15d | -0.11 ± 0.026c | 4.21 ± 0.136a | 45.53 ± 0.08d | 57.88 ± 0.29a | 2.12 ± 0.07d | 8.40 ± 0.10a | 42.14 ± 0.27a |
| Padet | 62.21 ± 0.33c | 1.05 ± 0.056a | 2.33 ± 0.475bc | 47.05 ± 0.34c | 47.96 ± 0.27c | 3.11 ± 0.06c | 3.63 ± 0.09d | 35.18 ± 0.16c |
| Tadese | 63.59 ± 0.20b | 0.78 ± 0.122b | 2.21 ± 0.090c | 48.32 ± 0.23b | 50.16 ± 0.32b | 3.73 ± 0.12b | 4.68 ± 0.17c | 35.96 ± 0.23b |
| a, b, c, and d superscripts are significantly (p < 0.05) different column wise within and between different millet cultivars, n=5. L*, a*, b* and ∆E represents degree of lightness, red-green, yellow-blue and total color difference, respectively. | ||||||||
Table 3: Color characteristics of millet flour and injera.
| Variety | L* | a* | b* | ∆E |
|---|---|---|---|---|
| Axum | 44.91 ± 0.60c | 7.97 ± 0.56b | 5.32 ± 0.73bc | 29.30 ± 0.62b |
| Kola-1 | 57.11 ± 2.18a | 1.68 ± 0.61d | 8.75 ± 1.37a | 41.85 ± 1.49a |
| Padet | 44.34 ± 0.31c | 6.92 ± 0.21c | 3.77 ± 0.19c | 30.08 ± 0.23b |
| Tadese | 46.40 ± 0.55c | 8.02 ± 0.59b | 6.47 ± 0.87b | 30.05 ± 0.28b |
| Tesema | 48.67 ± 0.84b | 10.44 ± 0.50a | 9.53 ± 0.89a | 29.71 ± 0.34b |
| a, b, c, and d superscripts are significantly (p<0.05) different column wise within and between different millet cultivars, n=5 | ||||
Table 4: Grain color characteristics.
Among finger millet cultivars, Tesema was statistically (p<0.05) varied and had the highest L*, a* and b* values. Axum and Tadese did not show significant difference in L*, a*, b* and ΔE values and Padet had the lowest L* and b* values.
The L* value of flours ranged from 59.76 to 64.69 and that of injera ranged from 47.96 to 57.88. Tesema and Kola-1, had a highest L* value from flours and injera, respectively. Except for Kola-1, the L* value of flours was higher, which indicated lighter color than Kola-1. The degree of the red-green colour of flours ranged from -0.11 to 1.66. The flours from finger millet varieties showed the maximum redness with non-significant (p<0.05) among them and conversely, Kola-1 was statistically differed and had the minimum redness and maximum green color. In addition, a significant difference was observed between flours of millet varieties in their value of yellow-blue color (b* value). Kola-1 attained maximum yellowness (p<0.05), and Tadese, Padet and Tesema showed more blueness with non- statistical (p>0.05) variation among them. This result is comparable to Yadav et al. reported hunter L*, a* and b* values of 79.3, 1.23 and 12.6, respectively for pearl millet flour [2]. Vishwanath et al. reported the L*, a*, b* and ΔE color values of 69.9, 2.6, 8.9 and 45.9 respectively, for whole finger millet flour [58].
The total color difference (ΔE) of flours and injera ranged between 45.53 to 48.85 and 35.18 to 42.14, with Tesema and Kola-1, respectively showing significantly (p<0.05) higher values than others. Axum and Padet were found to have similar ΔE value both in flours and injera. The colour of any food product generally changes during heat treatment and thus influences the acceptability. After fermentation and then baked to injera, L* value was significantly (p<0.05) reduced in comparison to flours (Figure 3). Kola-1 had the highest L* value than others. However, baking resulted in higher a* values indicating more redness with values ranging from 2.1 to 4.46. Except for Axum and Tadese, the b* value of injera was statistically different. The b* value was increased upon baking when compared with flours from all millet varieties and showed a maximum yellowness. These differences could be related to their chemical compositions particularly due to fat (help as an insulator), amino acids and reducing sugar contents that affect maillard reaction.
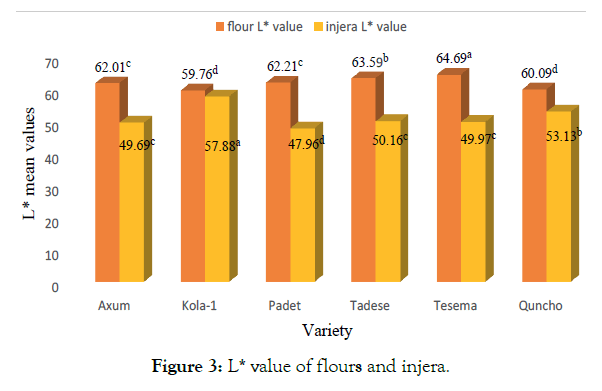
Figure 3:L* value of flours and injera.
The results agreed with Sandhu et al. observed the similar trend of decrease in L* value and increase in the both a* and b* values of flours from oat cultivars after toasting [59]. Siroha et al. revealed that L*, a* and b* values of toasted pearl millet cultivars ranged from 73.1 to 75.7, 0.36 to 1.76 and 11.1 to 14.2, respectively [60]. Siroha et al. reported ΔE for Indian pearl millet flours between 52.5 and 75.1 [38]. Yadav et al. reported hunter L*, a* and b* values of 79.3, 1.23 and 12.6, respectively for hydrothermally treated pearl millet flour [2]. Difference in L* value was probably due to polymerization of phenolics and pigments such as anthocyanins [61]. Heat processing may also have caused conversion of flavanols in colour pigment into intermediate compounds that might have caused slight change in colour of resulting flour [62]. In general, it was observed that baking had significantly (P<0.05) changed the colour of the flour in all the samples. The total color difference of millet grains and flours, and flours and injera was negatively correlated (r=-0.736; - 0.739) and were statistically different. However, grains and injera showed positive and strong correlation (r=0.99) (Figure 4).
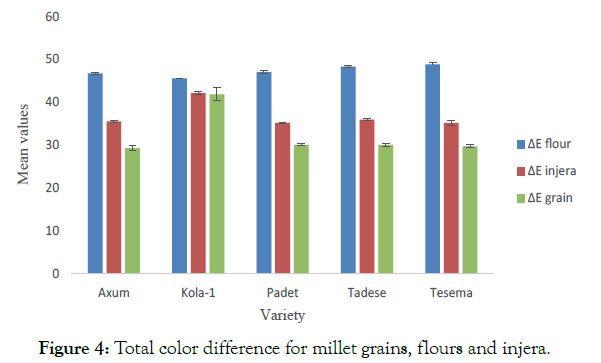
Figure 4:Total color difference for millet grains, flours and injera.
The rheological, functional and color characteristics directly and/ or indirectly affects product quality and quantity. This might be largely due to the inherent properties (chemical composition and nutritional quality) of the grains. Based on the result in this study, it can be concluded that the pearl millet variety, Kola-1 showed the lowest flour swelling power, pasting property profiles falling number, and L* and a* color values when compared to finger millets. Conversely, it had a highest gel consistency, flour solubility, grain color (L* and ΔE), flour color (b*) and injera color (L*, a* and ΔE). Among finger millet cultivars, Padet had highest pasting profiles and the lowest color characteristics of injera (L*, a*, and b* values).
The authors would like to gratefully acknowledge the VLIR and Can Tho University Improvement Project, VN14-P6 supported by Japanese ODA loan, and Can Tho University, for the financial and administrative support. In addition, we express our deep gratitude to Mr. Fayera and Dr. Habtamu Admassu, and their team for their kind assistance and permitting us to carry out the research work at Grain Quality Laboratory, Addis Ababa Science and technology University, Ethiopia.
Citation: Dasa F, Binh LN (2019) A Comparative Study on Rheological, Functional and Color Properties of Improved Millet Varieties and Injera. J Agri Sci Food Res. doi: 10.35248/2593-9173.19.10.267
Received: 10-Oct-2019 Accepted: 21-Oct-2019 Published: 28-Oct-2019 , DOI: 10.35248/2593-9173.19.10.267
Copyright: © 2019 Dasa F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.