Organic Chemistry: Current Research
Open Access
ISSN: 2161-0401
ISSN: 2161-0401
Research Article - (2015) Volume 4, Issue 2
Computational quantum chemistry was used to investigate the structures of lithium carbamates in the gas phase and in ethereal solvents. These compounds act as nucleophiles with either inversion or retention of configuration at the chiral center and knowledge of the aggregation state is the first step in understanding the reactivity. The sterically hindered lithium phenyl carbamate is calculated to exist largely as the ether or THF solvated monomer in solution. Higher aggregates are possible in the gas phase, which is often taken as an approximation for solutions in non-polar solvents.
Keywords: Lithium carbamates; Computational chemistry; Solution structures; Aggregation state
Enantioenriched organolithium compounds are highly useful intermediates in synthesis. Whilst chiral non-stabilized organolithium compounds almost always react with electrophiles with retention of configuration, the situation is less clear cut with mesomerically stabilised sp3 hybridized intermediates, for example benzylic carbamate 1 and indanyl carbamate 2, (Scheme 1). They are lithiated with s-BuLi in the presence of the chelating diamine TMEDA, and are configurationally stable at low temperatures.
Hoppe found that the stereochemical course of the substitution reaction with benzylic lithiated carbamate Li-1 was electrophile dependent e.g. CO2, MeOCOCl, Me3SiCl, R3SnCl and RNCO reacted with a high degree of inversion of configuration whereas proton acids, aldehydes, ketones, methyl carboxylates, and dimethylcarbonate react with retention of configuration (Scheme 2) [1,2]. It was concluded from these results that electrophiles usually prefer to react with inversion unless the electrophile can complex with the lithium cation in which case it is delivered on the same face of the metal and so reacts with retention. If the barrier to inversion is increased, as in the case of indanyl 2, the same electrophiles now react with a high degree of retention [3] (Table 1). Related reactions of 2,4,6-tri-ispropylbenzoates in place of carbamates were reported by Hammerschmidt with similar results although in this case acid chlorides reacted with retention rather than inversion [4,5].
| Aggregate | Conformation 1→2 | Conformation 2→3 |
|---|---|---|
| LiCH2OCON(CH3)2m | -23.7 | N/A |
| LiCH2OCON(CH3)2d1 | -21.6 | 1.92 |
| LiCH2OCON(CH3)2d2 | -5.29 | 0.764 |
| LiCHPhOCON(CH3)2m | -25.0 | N/A |
| LiCHPhOCON(CH3)2d1-RR | -2.15 | 1.52 |
| LiCHPhOCON(CH3)2d1-RS | -2.36 | 2.72 |
| LiCHPhOCON(CH3)2d2-RR | -14.0 | 11.4 |
| LiCHPhOCON(CH3)2d2-RS | -13.8 | 10.2 |
Table 1: Relative free energies (kcal/mol) of N-lithium and O-lithium coordination of lithium carbamate monomers and dimers.
In a more dramatic example, we showed that boranes and boronic esters reacted with benzylic lithiated carbamate Li-1 with very high and diametrically opposite selectivity: boranes with inversion and boronic esters with retention (Scheme 3). In the case of the indanyl substrate 2 the degree of retention was high when boronic esters were used, but retention began to become competitive even with boranes, leading to a low enantioenrichment of the product alcohols [6].
Lithiation-borylation can also be undertaken with triisopropylbenzoate esters such similar to those developed by Hammerschmidt. Lithiation of 1,3,5-triisopropylbenzoate ester [7,8] 3 with s-BuLi in the presence of (-)-sparteine gives lithiated species Li-3 (Scheme 4). Trapping with pinacol boronic ester occurs with retention of stereochemistry, and 1,2-metallate rearrangement followed by oxidation gives secondary alcohols with overall retention of stereochemistry.
The scope and complexity of these reactions makes it necessary to understand the structures of the reactants in solution. Structural features include both aggregate and solvent effects. Aggregation of organolithium compounds has been known since the 1980’s, with tetramers or higher aggregates of alkyllithiums being common in nonpolar solvents, and monomers, dimers and tetramers being common in non-polar solvents [8]. Lithium carbenoids and structurally similar compounds are generally aggregated in solution [9-16]. Alkyllithiums with an electronegative atom attached to the lithium-bearing carbon have properties ranging from those of carbenes to stabilized carbanions. In the lithium carbamates of this study, the oxygen lone pair effects on the C-Li group are attenuated by the attached carbonyl group. The behaviour of these compounds is more carbanion-like inspite of the structural similarity to α-lithioethers. The latter compounds have intermediate carbene-carbanion like properties. In this paper we used computational methods to better understand the structures of these useful intermediates in the solvated and unsolvated (gas phase) states.
All geometry optimizations and frequency calculations were performed with the Gaussian 09 program [17]. Geometry optimizations were performed at the M06/6-31+G(d) [18] level of theory, followed by frequency calculations at the same level. Vibrational frequencies calculated at 298.15 K and the thermal energies to the free energies, obtained from the frequencies, were added to the electronic energies at each level of theory, in order to obtain approximate free energies of each species.
Solvent effects were modelled by placing explicit diethyl ether or THF ligands on the lithium atoms. One or two ligands were placed on each lithium atom according to the structure and the number of ligands that fit without causing excessive steric strain. Special care is taken to ensure consistent handling of standard states [19,20]. Specifically, a correction term RTln (c°RT/P°) must be added per mole of each species in the reaction under consideration, which represents the change in free energy involved in compressing the system from standard pressure P° (or a concentration of P°/RT) used in gas phase calculations to the standard concentration of c°=1 mol/L commonly used for solutions. This term is numerically equal to +1.8900 kcal/mol at 298.15 K. While it cancels from both sides when the net change in the number of moles due to reaction Δn=0, it is a non-negligible correction in cases where Δn ≠ 0. Yet another correction is required for cases where a THF or ether ligand dissociates, illustrated for THF by:
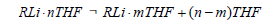
for which
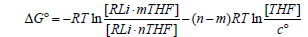 (1)
(1)
Since the concentration of pure THF or ether is different from the one M standard concentration c°, it was evaluated from its molar volume at 1 atm and 298.15 K using the empirical expression provided by Govender and coworkers [21] and incorporated into the second term of Equation (1). Numerically, this correction to ΔG° amounts to -1.4883 kcal/mol per THF and -1.3360 kcal/mol per diethyl ether at 298.15 K. This approach to modeling solvation effects on organolithium compounds has been used previously [22-28] and has been found to give results in good agreement with available experimental results.
The simplest of the lithium carbamates is an achiral compound LiCH2OCON(CH3)2. It could potentially exist as a monomer, dimer, tetramer, or hexamer by analogy to alkyllithiums and other lithium carbenoids and carbenoid-like compounds. Furthermore, different structures are possible for some of those aggregates. Analogous structures are formed by the chiral LiCHPhOCON(CH3)2 lithium phenyl carbamates. The gas phase monomer can exist as two different conformational isomers, shown in Figure 1. Conformation 1 has the lithium atom coordinated to an oxygen and a nitrogen atom, while in conformation 2, the lithium atom is coordinated to both oxygen atoms.
The lithium carbamate dimer exists as two regioisomers. The first, designated as Isomer 1, connects the C-O-Li-C-O-Li atoms around a six-membered ring. The other regioisomer, designated Isomer 2, connects the atoms in the order C-Li-C-O-Li-O. Each of those regioisomers exists in 3 conformations. In conformation 1, the both lithium atoms are coordinated to nitrogen in addition to the oxygen in the 6 member ring. In conformation 2 both lithiums are coordinated to the carbonyl group’s oxygen atom. In conformation 3 both types of lithium coordination are found. These six structures are shown for Isomer 1 and Isomer 2 in (Figures 2 and 3) respectively.
The relative energies of the various gas phase isomers and conformations are shown in (Table 1). The LiCH2OCON(CH3)2 and LiCHPhOCON(CH3)2 monomers both exist almost exclusively in the oxygen coordinated conformation (conformation 2) as indicated by the free energy change of more than -20 kcal/mol. This preference for oxygen coordination was also seen in the dimers. Nitrogen coordination in the dimers in conformations 1 and 3 is much weaker or non-existent, compared to the monomers. In the case of the LiCHPhOCON(CH3)2 dimer, conformation 1 is also more sterically hindered due to the interaction between the two phenyl groups, leading to additional stabilization of conformation 2.
The LiCH2OCON(CH3)2 can potentially exist as a tetramer or hexamer, as shown in (Figure 4). The additional steric strain of the phenyl group prevents the analogous aggregates of LiCHPhOCON(CH3)2 from forming. Starting from the monomer and the most stable conformation of each dimer, the calculated aggregation energies of the gas phase LiCH2OCON(CH3)2 and LiCHPhOCON(CH3)2 species are shown in (Table 2). Dimer formation from the LiCH2OCON(CH3)2 monomer is slightly exergonic, but the tetramer is the most favoured aggregate, apparently from a balance between maximum lithium coordination and low steric strain. In contrast, LiCHPhOCON(CH3)2 is predicted to exist almost exclusively as dimer 2 in the gas phase.
| Licarbamate | 2 m→d1 | d1→d2 | 2 d1→tet | 3/2 tet→hex |
|---|---|---|---|---|
| LiCH2OCON(CH3)2 | -0.398 | 2.40 | -22.0 | 21.3 |
| LiCHPhOCON(CH3)2 RR | 1.07 | -9.83 | N/A | N/A |
| LiCHPhOCON(CH3)2 RS | 1.31 | -8.29 | N/A | N/A |
Table 2: Calculated gas phase aggregation free energies (kcal/mol) of LiCH 2OCON(CH3)2 and LiCHPhOCON(CH3)2.
From these results, it is seen that the gas phase carbamates exist as well defined aggregates with nitrogen and/or oxygen coordinated to the lithium atoms in a well-defined manner. That is not always the case for solvated species, as is seen from the discussion below. Solvation was modelled using diethyl ether and THF as solvating ligands, as they are the two most common solvents used in synthetic chemistry involving these and similar organolithium compounds. Figure 5 shows the optimized geometries of diethyl ether and THF solvated monomers in the two conformations analogous to the gas phase structures.
Comparison of Figures 1 and 5 shows the similarity of the unsolvated and solvated monomer structures. The energies in Table 3 show that the diethyl ether disolvates exist primarily in the disolvated form. The stronger coordination to THF is reflected in the third solvation energies, which still favour the disolvate but less so than with diethyl ether solvation. The energies in Table 4 show that for both the disolvated and trisolvated monomers, Conformation 2, with the lithium atom coordinated to oxygen instead of nitrogen, is favoured by between 16 and 22 kcal/mol, making it the predominant monomer conformation in both the gas phase and in ethereal solvents.
| Aggregate | 3rd Et2O | 4th Et2O | 3rdTHF | 4th THF |
|---|---|---|---|---|
| LiCH2OCON(CH3)2m conf 1 | 4.85 | N/A | 0.359 | N/A |
| LiCH2OCON(CH3)2m conf 2 | 7.49 | N/A | 2.06 | N/A |
| LiCH2OCON(CH3)2d1 conf 1 | -1.50 | 0.604 | -12.1a | 2.40 |
| LiCH2OCON(CH3)2d1 conf 2 | 23.8 | 0.799 | -4.24 | -0.415 |
| LiCH2OCON(CH3)2d1 conf 3 | 13.5 | 3.84 | -0.119 | 0.709 |
| LiCH2OCON(CH3)2d2 conf 1 | -3.72 | -0.0403 | -17.0 | 7.33 |
| LiCH2OCON(CH3)2d2 conf 2 | -1.66 | 4.01 | 21.9b | -8.34 |
| LiCH2OCON(CH3)2d2 conf 3 | -0.794 | 8.56c | -5.48 | 3.43 |
| LiCHPhOCON(CH3)2m conf 1 | 7.08 | N/A | 2.41 | N/A |
| LiCHPhOCON(CH3)2m conf 2 | 9.79 | N/A | 1.83 | N/A |
| LiCHPhOCON(CH3)2d1 RR conf 1 | d | d | d | d |
| LiCHPhOCON(CH3)2d1 RS conf 1 | d | d | d | d |
| LiCHPhOCON(CH3)2d1 RR conf 2 | 3.24 | 1.34 | -0.206 | -2.71 |
| LiCHPhOCON(CH3)2d1 RS conf 2 | 5.77 | -3.94 | -0.820 | -4.81 |
| LiCHPhOCON(CH3)2d1 RR conf 3 | d | d | d | d |
| LiCHPhOCON(CH3)2d1 RS conf 3 | d | d | d | d |
| LiCHPhOCON(CH3)2d2 RR conf 1 | d | d | d | d |
| LiCHPhOCON(CH3)2d2 RS conf 1 | d | d | d | d |
| LiCHPhOCON(CH3)2d2 RR conf 2 | d | d | d | d |
| LiCHPhOCON(CH3)2d2 RS conf 2 | d | d | d | d |
| LiCHPhOCON(CH3)2d2 RR conf 3 | d | d | d | d |
| LiCHPhOCON(CH3)2d2 RS conf 3 | d | d | d | d |
(a) Stronger binding THF forced conformational change resembling conformation 2.
(b) Stronger binding THF forced conformational change resulting in more strain.
(c) 4th ether dissociated.
(d) These solvates were too strained to exist.
Table 3: Free energies (kcal/mol) of successive solvation of lithium carbamate monomers and dimers.
| Aggregate | Conformation 1→2 | Conformation 2→3 |
|---|---|---|
| LiCH2OCON(CH3)2m•2Et2O | -20.7 | N/A |
| LiCH2OCON(CH3)2m•3Et2O | -17.0 | N/A |
| LiCH2OCON(CH3)2m•2THF | -18.3 | N/A |
| LiCH2OCON(CH3)2m•3THF | -16.6 | N/A |
| LiCH2OCON(CH3)2d1•2Et2O | -21.6 | 1.92 |
| LiCH2OCON(CH3)2d1•3Et2O | 3.70 | -9.73 |
| LiCH2OCON(CH3)2d1•4Et2O | 3.90 | -6.67 |
| LiCH2OCON(CH3)2d1•2THF | 4.77 | -13.9 |
| LiCH2OCON(CH3)2d1•3THF | 11.2 | -9.75 |
| LiCH2OCON(CH3)2d1•4THF | 8.35 | -8.63 |
| LiCH2OCON(CH3)2d2•2Et2O | 0.858 | -13.3 |
| LiCH2OCON(CH3)2d2•3Et2O | 2.92 | -12.5 |
| LiCH2OCON(CH3)2d2•4Et2O | 6.97 | -7.94 |
| LiCH2OCON(CH3)2d2•2THF | -22.3 | 11.2 |
| LiCH2OCON(CH3)2d2•3THF | 16.6 | -16.2 |
| LiCH2OCON(CH3)2d2•4THF | 0.972 | -4.46 |
| LiCHPhOCON(CH3)2m•2Et2O | -21.7 | N/A |
| LiCHPhOCON(CH3)2m•3Et2O | -19.5 | N/A |
| LiCHPhOCON(CH3)2m•2THF | -19.5 | N/A |
| LiCHPhOCON(CH3)2m•3THF | -20.1 | N/A |
| LiCHPhOCON(CH3)2d1 RR•2Et2O | 4.06 | 1.54 |
| LiCHPhOCON(CH3)2d1 RR•2THF | 4.79 | -0.176 |
| LiCHPhOCON(CH3)2d1 RS•2Et2O | -5.99 | -1.43 |
| LiCHPhOCON(CH3)2d1 RS•2Et2O | 2.53 | -6.45 |
| LiCHPhOCON(CH3)2d2 RR•2Et2O | -9.84 | 1.47 |
| LiCHPhOCON(CH3)2d2 RR•2THF | -8.24 | 5.29 |
| LiCHPhOCON(CH3)2d2 RS•2Et2O | -14.0 | 4.76 |
| LiCHPhOCON(CH3)2d2 RS•2Et2O | -10.2 | 3.55 |
Table 4: Relative free energies (kcal/mol) of N-lithium and O-lithium coordination of lithium carbamate monomers and dimers in diethyl ether and THF.
The LiCH2-carbamate dimers can exist in 3 different conformations and as the di-, tri, and tetrasolvates, for a total of 9 possible solvated structures for each solvent. The optimized geometries of Dimer-1 are shown in Figure 6.
The structures of the solvated LiCH2-carbamate dimers (Dimer 1) are roughly similar to the gas phase dimers, except that some of the internal Li-O coordination is disrupted by the ether and THF ligands. That is most apparent in Conformations 2 and 3 in the tri- and tetrasolvated forms. The calculated third and fourth solvation energies in Table 3 show that Conformations 2 and 3 strongly resist adding a third ether solvent ligand due to steric crowding. The trisolvated form of Conformation 1 is also favoured, although small amounts of the di- and tetrasolvated forms may exist. THF is a stronger coordinating solvent than diethyl ether and the calculated energies show a modest tendency of Conformations 2 and 3 to undergo further solvation. Conformation 1 is most stable in the THF trisolvated form, as the stronger coordination of THF caused a conformational change which more closely resembled conformation 2.
The diethyl ether solvated carbamates were calculated to exist primarily as the disolvates, and the data in Table 4 shows that Conformations 2 and 3 are both favoured over Conformation 1. In the ether tri- and tetrasolvated forms Conformation 3 is favoured.
The more strongly coordinating THF ligand successfully competes for lithium coordination with the carbamate oxygen atoms, forcing conformational changes to accommodate the third and fourth ligands. The data in Table 4 show that Conformation 3 is favoured in the THF di- and tetrasolvated forms, while the trisolvate has a modest preference for Conformation 1. These conformations appear to achieve the best balance between internal oxygen and THF coordination to the lithium atoms.
The LiCHPh-carbamate exists as two possible diastereomers (2 pairs of enantiomers). Since both enantiomers are of equal energy, one the structure of one enantiomer from each pair, the RR and RS were calculated, and the optimized geometries are shown in Figure 7 for the diethyl ether solvates and in Figure 8 for the THF solvates. The additional steric strain of the phenyl group inhibits the formation of the tri-and tetrasolvates of Conformations 1 and 3 with diethyl ether, and forcing Conformation 2 into a less strained form with reduced lithium coordination to the carbamate oxygen atoms. Even in Conformation 2, the energy of the third ether coordination was endergonic. In the RS isomer, the structure change upon adding the third ether facilitated the fourth ether coordination, as shown in Table 3.
A similar situation was found with the third and fourth THF ligands. The trisolvate and tetrasolvate were found only for Conformation 2. The data in Table 3 show that for both the RR and RS isomers, the tetrasolvate is the most stable.
The most stable forms of the disolvated LiCH2OCON(CH3)2 dimer-2 had both solvent ligands on the same lithium atom. That minimized the disruption of internal oxygen coordination to the other lithium atom, making the disolvated structures quite similar to the gas phase structures, as shown in Figure 9. Up to four ligands could be accommodated (2 per lithium) but addition of the third or fourth ligand sometimes resulted in significant conformational changes, so that the names Conformation 1, Conformation 2, and Conformation 3 become blurred in the higher solvations states. The energies in Table 3 show the effects of the additional steric strain on the ability to accommodate the full set of 4 ether or THF ligands. With the diethyl ether solvates Conformation 3 is favoured according to the relative energies listed in Table 4. The THF disolvated structure is most stable in Conformation 2 but that conformation is too sterically hindered to be favoured as the tri-or tetrasolvate.
The more hindered LiCHPhOCON(CH3)2 dimer-2 could only accommodate two ether or THF ligands, one on each lithium atom. Attempts to add additional ligands resulted in dissociation of the third ligand during the geometry optimization. The optimized structures are shown in Figure 10. The data in Table 4 show that Conformation 2 is energetically favoured in both the RR and RS isomer, for both the ether and THF solvates.
The data in Table 5 compares the relative stability of the two dimers in the various possible solvation states. For the LiCH2OCON(CH3)2 carbamate Dimer 1 is strongly favoured in the ether disolvate, and either slightly favoured or disfavoured in higher solvated forms. The THF disolvate favors Dimer 2. In pure ethereal solvents, a mixture of solvation states will likely result in a mixture of the two dimers. However, Dimer 1 could potentially be favoured by preparing the carbamate in a hydrocarbon solvent containing a limited amount of ether, and Dimer 2 could be likewise favoured in THF. The LiCHPhOCON(CH3)2 carbamates, existing only as the disolvates, show a modest preference for Dimer 2 in both diethyl ether and THF.
| Lithium Carbamate/Solvation State | Dimer 1→Dimer 2 |
|---|---|
| LiCH2OCON(CH3)2•2Et2O | 10.9 |
| LiCH2OCON(CH3)2•3Et2O | -3.92 |
| LiCH2OCON(CH3)2•4Et2O | 0.800 |
| LiCH2OCON(CH3)2•2THF | -10.1 |
| LiCH2OCON(CH3)2•3THF | -1.80 |
| LiCH2OCON(CH3)2•4THF | -0.0809 |
| LiCHPhOCON(CH3)2•2Et2O RR | -0.802 |
| LiCHPhOCON(CH3)2•2Et2O RS | -2.01 |
| LiCHPhOCON(CH3)2•2THF RR | -1.47 |
| LiCHPhOCON(CH3)2•2THF RS | -2.51 |
Table 5: Relative energies of Dimers 1 and 2 (Dimer 1 → Dimer 2, kcal/mol). Most stable conformation of Dimer 1 compared to most stable conformation of Dimer 2.
We have shown that the solvent can have a significant effect on the aggregation state and internal coordination of these lithium carbamamtes.
The unresolved question at this point is whether these lithium carbamates exist as dimers, or whether they are among the relatively small group of lithium compounds existing as monomers in solution. That question is resolved by the data in Table 6. Although the unhindered LiCH2OCON(CH3)2 carbamate can exist as the dimer in ether, the stereochemically interesting LiCHPhOCON(CH3)2 carbamate exists exclusively as the monomer in both solvents. The presence of a single aggregate, existing primarily in a single conformation due to lithium coordination, will greatly simplify the study of reaction mechanisms in which the chirality is either inverted or retained.
| Li Carbamate | Solvent | DG dimerization |
|---|---|---|
| LiCH2OCON(CH3)2 | Et2O | 5.06 |
| LiCH2OCON(CH3)2 | THF | -3.81 |
| LiCHPhOCON(CH3)2 RR | Et2O | 16.6 |
| LiCHPhOCON(CH3)2 RR | THF | 16.7 |
| LiCHPhOCON(CH3)2 RS | Et2O | 16.8 |
| LiCHPhOCON(CH3)2 RS | THF | 18.8 |
Table 6: Dimerization energies (based on most stable conformation of Dimer 2) of lithium carbamates (kcal/mol).
The aggregation state of the lithium carbamates described above depends on both steric strain and the solvent. In the gas phase, the LiCH2OCON(CH3)2 carbamate forms a tetramer, but that aggregate is not possible for the more hindered LiCHPhOCON(CH3)2 carbamate, which exists as a gas phase dimer. In ether and THF solution, steric strain disvavors the dimer from forming, making the LiCHPhOCON(CH3)2 carbamate one of a relatively few organolithiums to exist primarily as a monomer in solution.
This work was supported by National Science Foundation grant #CHE-1049622. This work utilized the resources of the National Energy Research Center (NERSC), which is supported by the Office of Science of the Department of Energy under Contract No. DE-AC02- 05CH11231. Special thanks to Varinder Aggarwal of the University of Bristol for providing background information, Schemes 1-3, and for helpful discussions.