Journal of Cell Signaling
Open Access
ISSN: 2576-1471
ISSN: 2576-1471
Short Communication - (2023)Volume 8, Issue 3
Spaceflight is associated with many physiological, environmental and psychological stress factors that can have considerable effects on the health of astronauts [1]. One of the most significant physiological challenges is Aerospace Microgravity (AMG)- induced inhibition of osteoblast differentiation and subsequent bone loss. Simulated Microgravity (SMG), a ground-based method that mimics AMG conditions, was developed to elucidate molecular mechanisms of AMG-altered osteoblast differentiation. It has been shown that SMG suppresses osteoblast differentiation and promotes bone loss via the Wnt/β- catenin pathway in a similar fashion to patients with osteoporosis [2,3]. Nonetheless, the mechanism by which SMG alters Wnt/ β- catenin signaling is unknown.
Cell surface integrins that interact with the extracellular matrix at the cell membrane form focal adhesions and act as the cell’s sensing system for external stimulatory signals [4]. Focal adhesion complexes are composed of various macromolecules that are localized at the focal adhesion site, such as α- and β-integrin transmembrane receptors, Intracellular Adaptor Proteins (IAPs) including scaffold vinculin, talin, and paxillin and IAP-recruited signaling molecules Focal Adhesion Kinase (FAK) and Ras Homolog gene (Rho) family GTPases RhoA, cell division-control protein-42 (Cdc42), and ras-related C3 botulinum-toxin substrate-1 (Rac1) [5]. As part of the focal adhesion complex, FAK functions as a scaffold for other focal adhesion components and as an important signaling initiator for other pathways [6-8].
We previously used a random positional 3D clinostat to model SMG for investigation of its effect on tumor cell biology. We determined that SMG inhibits tumor cell proliferation, migration and invasiveness, and promotes tumor cell apoptosis via FAK-regulated mammalian Target Of Rapamycin Complex-1 (mTORC1), AMP-activated Protein Kinase (AMPK), and Extracellular Signal-Regulated Kinase-1/2 (ERK1/2) pathways [9,10]. We thus hypothesized that FAK may also play a role in SMG-induced inhibition of Wnt/β-catenin-controlled osteoblast differentiation.
To test our hypothesis, we investigated the effect of SMG on osteoblast differentiation by using the mouse osteoblast cell line Murine Calvarial cell (MC3T3-E1) and 3D clinostat modeling. We observed that MC3T3-E1 cells cultured under SMG exhibit reduced formation of focal adhesions, altered cytoskeleton structure and down-regulated FAK signaling, compared to cells cultured under normal ground conditions [11]. SMG was also shown to down-regulate expression of Wnt-regulated transcription factor β-catenin and two markers of osteoblastic maturation: Bone Morphogenetic Protein-2 (BMP-2) and type-1 Collagen (COL-1). Further, SMG inhibited Alkaline Phosphatase (ALP) and matrix mineralization activity, which are two major measurable parameters of osteoblast function [11]. Next, in a mouse Hind-limb Unloading (HU) model that mimics an in vivo SMG condition, we found decreased trabecular bone volume, thinning or loss of trabeculae in the proximal tibia, compared to control mouse tibia assessed by micro-computed tomography imaging [11]. The tibial bone loss in mice that underwent HU treatment was then confirmed by histological analyses [11]. Lastly, Cytotoxic Necrotizing Factor-1 (CNF-1) an E. coli toxin is a broad-spectrum activator of RhoA and FAK [11-13] and also a well-tolerated pharmaceutical treatment for central nervous system diseases in rodents [13,14]. Interestingly, we found that the FAK activator, CNF-1, counteracts the above alterations in focal adhesion assembly, cytoskeleton structure, expression of various genes and osteoblast differentiation in SMG-treated MC3T3-E1 cells as well as bone density in HU-treated mice [11]. Altogether, these findings indicate that FAK is a critical molecular signaling that restores SMG-inhibited osteoblast differentiation via the FAK-Wnt/ β-Catenin-BMP-2/COL-1 pathway (Figure 1).
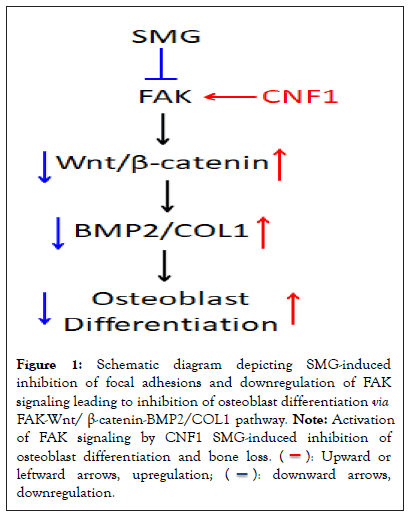
Figure 1: Schematic diagram depicting SMG-induced
inhibition of focal adhesions and downregulation of FAK
signaling leading to inhibition of osteoblast differentiation via FAK-Wnt/ β-catenin-BMP2/COL1 pathway. Note: Activation
of FAK signaling by CNF1 SMG-induced inhibition of
osteoblast differentiation and bone loss.  leftward arrows, upregulation;
leftward arrows, upregulation;  downregulation.
downregulation.
In summary, we demonstrated that FAK signaling plays an important role in SMG-induced inhibition of osteoblast differentiation via the FAK-Wnt/β-catenin-BMP2/COL1 pathway, and that activation of FAK by CNF1 successfully counteracts SMG-induced inhibition of osteoblast differentiation and restores bone formation in HU-treated mice. Therefore, it may be worthwhile to explore the FAK-Wnt/β-catenin-BMP2/ COL1 pathway as a new target in the development of novel therapeutics for astronauts at risk of osteoblast differentiation inhibition and bone loss, and for clinic patients with osteoporosis.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Yu M, Xiang J (2023) A Critical Signaling Focal Adhesion Kinase (FAK) Restoration Simulated Microgravity-Induced Inhibition of Osteoblast Differentiation via FAK-WNT/?-Catenin-Bmp2/Col1 Pathway. J Cell Signal.08:347.
Received: 08-Jun-2023, Manuscript No. JCS-23-25191; Editor assigned: 12-Jun-2023, Pre QC No. JCS-23-25191 (PQ); Reviewed: 26-Jun-2023, QC No. JCS-23-25191; Revised: 03-Jun-2023, Manuscript No. JCS-23-25191 (R); Published: 29-Sep-2023 , DOI: 10.4172/ 2576-1471.23.8.347
Copyright: © 2023 Yu M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.