Journal of Clinical & Experimental Dermatology Research
Open Access
ISSN: 2155-9554
ISSN: 2155-9554
Research Article - (2020)Volume 11, Issue 4
Background: Available data suggests that the manifestation of aging has a strong genetic basis, which can modify an
individual ‘ s susceptibility to specific skin aging signs. Proteins such as matrix metalloproteinases, aquaporins,
filaggrin, superoxide dismutase and glutathione peroxidase have specific roles. Their encoding genes present single
nucleotide polymorphisms resulting in different responses to skin aging for elasticity, hydration, barrier function and
wrinkles.
Aim: This study aimed to investigate the anti-ageing and anti-oxidant efficacy of a skin care regimen designed to
address the specific needs of a certain genetic risk profile: high risk for collagen breakdown, medium risk for antioxidant
production and low risk for dehydration and impaired barrier function.
Methods: DNA samples of 100 participants were collected for genetic profile analysis. Of these, 24 participants
presenting the most abundant genetic risk profile were enrolled on a 56 days anti-aging efficacy study of a combined
treatment. The antioxidant efficacy of one investigational product was assessed for 14 participants.
Results: Significant wrinkle’s depth and skin roughness improvements were found for the investigational treatment
in comparison to the comparator and baseline. No variations were observed for the skin hydration and barrier
function when compared to the comparator. The skin serum provided a significant antioxidant efficacy up to 24 h.
Conclusion: A skin care regimen designed to address the specific needs of a genetic risk profile characterized by high
risk for collagen breakdown and medium risk for low anti- oxidant production was effective on decreasing wrinkles,
improving skin roughness and protecting the skin from UV oxidative damage.
Genomic; Cosmetics; Anti-ageing; Personalized treatment; SNPs; Efficacy testing
In the last decade, the understanding of how DNA and RNA influence phenotype, organismal aging, and response to the environment has made great strides. Scientists have transformed this new information into treatments and therapies, leading to a closer link between the laboratory bench and the patient ’ s bedside. Genomic medicine is an emerging discipline that involves using genomic information about an individual to allow for personalized treatment, and is a rapidly advancing field of health care that can take into account every person’s unique clinical, genetic, genomic, and environmental information [1]. As medicine begins to embrace genomic tools that enable more precise prediction and treatment for disease, the cosmetic industry is also beginning to explore the benefits of a more personalized treatment based on genomic profiles.
Ageing is a process affecting the entire body and can be driven by both extrinsic and intrinsic factors, reflecting the influence of environmental versus genetic factors in the variability of characteristic phenotypic markers [2,3]. Because it ’ s the outermost barrier between the environment and the human body, skin changes are among the most visible signs of ageing and properties such as hydration, elasticity, and antioxidant capacity play a key role in the skin ageing process [4,5].
In recent years, scientists have found that certain genes can play important roles in determining the rate of ageing in model organisms [6]. Recent studies on twins have revealed that up to 60% of the skin ageing variation between individuals can be attributed to genetic elements, while the remaining 40% are related to non-genetic factors [5]. The use of genetic signatures for the identification of skin care individual necessities opens the door to personalized treatments. With genotypic services becoming increasingly more affordable, this resource could be a reality in the not-so-distant future. Directions for further research include the discovery of new proteins associated with skin aging, additional polymorphisms that modify their activity or expression, and understanding epigenetic modifications of DNA that affect gene expression. One can even envisage having the complete DNA sequence of an individual available as an aid to design a personalized skin care and anti-aging treatments.
Recent advances in this research field have led to the association of certain single nucleotide polymorphisms (SNPs) to skin properties. SNPs are the most common type of genomic variation among people, each SNP representing a single nucleotide difference, which could be a substitution or deletion. Each SNP may result in an amino acid change at a specific position and a different protein production. Researchers have found SNPs that may help predict an individual’s response to certain drugs, susceptibility to environmental factors such as toxins, and risk of developing particular diseases [5,7]. It is generally believed that the complete human sequence will reveal at least a million SNPs in non-repetitive sequences of coding regions, including introns and promoters.
Naval et al. [5] identified 13 SNPs in genes coding for proteins that play an important role in skin properties associated with ageing, namely, oxidative stress, elasticity, and hydration. SNP analysis of the subjects was used to classify 120 female volunteers into ten genotypic groups or clusters. The phenotype characteristics implied by known SNP properties was used to predict biochemical and metabolic properties of the skin. This clustering analysis suggests that different skin care necessities depend on the naturally occurring single genetic variants present in each one of the genetic clusters [5].
With this in mind, this investigation aimed to study the efficacy of a skin care regimen designed for a certain genetic skin ageing risk profile.
The Matrix metalloproteinase (MMP) genes are involved in the breakdown of extracellular matrix in normal physiological processes, such as embryonic development, reproduction, and tissue remodelling, as well as in disease processes. SNPs in MMP-1 (collagenase) and MMP-3 (stromelysin-1) are found to result in overexpression of the protein, potentially leading to increased breakdown of collagen and other structural proteins. Depending on the amount of SNPs present within this ‘collagen cluster’, we can assign a low, medium or high risk for accelerated collagen breakdown, influencing the ageing phenotype [8,9].
Aquaporins (AQPs) and the filaggrin (FLG) gene: AQPs are proteins that facilitate the transport of water across cell membranes and filaggrin plays an important role in the skin's barrier function bringing structural proteins together in the outermost skin cells to form tight bundles, flattening and strengthening the cells to create a strong barrier. In addition, the processing of filaggrin proteins leads to production of molecules that are part of the skin's "natural moisturizing factor," helping to maintain hydration of the skin. SNPs in the respective genes result in decreased protein expression, therefore increasing the risk for a dehydration and compromised barrier [10,11]. Risk profiles (i.e. low, medium, high) are assigned depending on the type and amount of SNPs present within the genes of this ‘hydration cluster’.
Superoxide dismutase (SOD) and glutathione peroxidase (GPX) are enzymatic antioxidants that play an important role in fighting oxidative stress and free radicals [12]. Extrinsic factors such as exposure to UV light and smoking generate free radicals and have a negative impact on skin ageing. Molecules like proteins, lipids and DNA are the most exposed to oxidative damage which can interfere with their normal function and lead to several pathological processes, such as inflammation and cancer. The topical application of antioxidant products can neutralize these free radicals, leading to the reduction or prevention of the signs of skin aging and damage caused by UV radiation and erythema due to inflammation [13]. The amount to which your body or skin can fight free radicals depends on the levels of antioxidants produced. SNPs present in these genes results in a decreased activity of the respective enzymes and depending on the amount of SNPs present, we assign a ‘low’, ‘medium’ or ‘high’ risk to the ‘antioxidant cluster’ [12,14,15]. Several studies demonstrated that formulations with antioxidant compounds applied topically had potential against erythematous process [13-16].
This study aimed to investigate the efficacy of active ingredients designed to address the specific needs of a certain genetic risk profile: high risk for collagen breakdown, medium risk for antioxidant production and low risk for dehydration and impaired barrier function. In this study, active ingredients were incorporated into four skin care formulations to be used in combination in order to determine whether better results are obtained with skin care products personalized towards a specific genetic risk profile when compared to a simple cream without active ingredients (comparator product) (Figure 1).
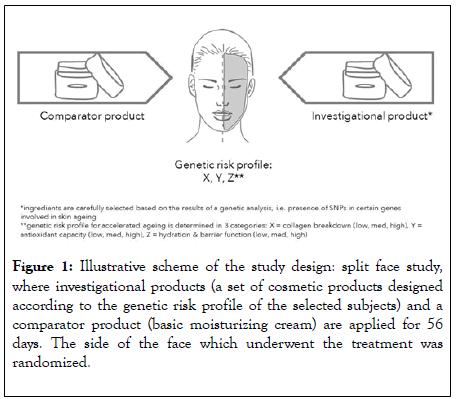
Figure 1: Illustrative scheme of the study design: split face study, where investigational products (a set of cosmetic products designed according to the genetic risk profile of the selected subjects) and a comparator product (basic moisturizing cream) are applied for 56 days. The side of the face which underwent the treatment was randomized.
Stage 1
Enrolment of subjects and DNA sample collection: Subjects were selected according to the following criteria: female; presenting a skin Fitzpatrick phototype I to III; between 30 and 65 years old; not pregnant or intending to conceive during the study; not breast-feeding; not having diseases that can interfere with the study; willing to participate in an anti-wrinkle in vivo study and presenting visible wrinkles on eye contour.
Saliva from 100 subjects was collected. For sample collection, subjects refrained from eating or drinking for 30 minutes prior to placing a swab into the subject’s mouth and brushing firmly against the inner cheek in a circular motion for approximately 30 seconds and then repeating the process on the other cheek. Finally, the swab was swept around the gum line at the bottom and around top of the mouth, taking care to avoid the tongue and teeth. After the sample collection, the swab was gently placed inside its individual transportation package and a second swab was used on the same subject, following the same protocol.
All samples (n=200) were dispatched for DNA analysis and the following genes were analysed for the presence of single nucleotide polymorphisms: MMP-1, MMP-3, AQP3, FLG, SOD2 and GPX which are biomarkers in 3 important biological processes involved in skin ageing. Certain SNPs in these genes influence the corresponding protein product expression or functionality, although none of them are monogenetic markers of pathology. We cluster these genes into 3 biological pathways or processes involved in the ageing process: collagen breakdown (MMP-1 and MMP-3), antioxidant capacity (SOD2 and GPX) and hydration and barrier function (AQP3 and FLG). SNP analysis reveals the presence of mutations. Depending on the presence or absence of SNPs, subjects were classified into risk categories (low, medium, high) per cluster, according to our own protected algorithm. We want to investigate if medium or highrisk profiles will benefit from a targeted skin care approach, where the ingredients are specifically selected to compensate their genetic “flaws”.
A group of 24 individuals presenting the following profile was enrolled: high risk for collagen breakdown (because of SNP’s in MMP genes that could increase their activity), medium risk for anti-oxidant production (because of SNP’s in SOD2 and GPX due to which only 1 copy is ‘ functional ’ ) and low risk for dehydration and impaired barrier function (absence of SNP’s in AQP3 and FLG) (Figure 2); because this was the most abundant profile identified.
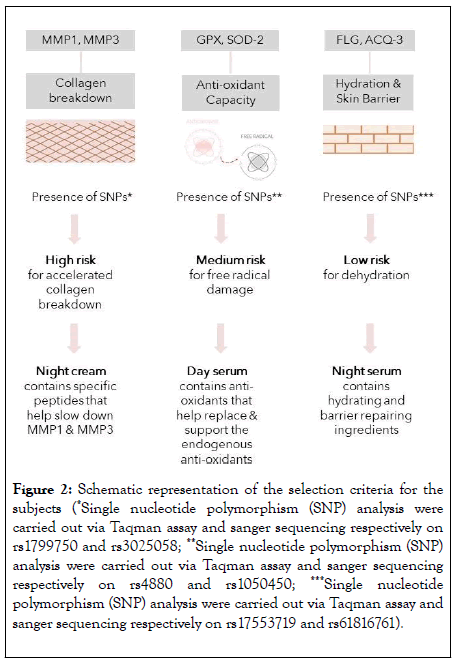
Figure 2: Schematic representation of the selection criteria for the subjects (*Single nucleotide polymorphism (SNP) analysis were carried out via Taqman assay and sanger sequencing respectively on rs1799750 and rs3025058; **Single nucleotide polymorphism (SNP) analysis were carried out via Taqman assay and sanger sequencing respectively on rs4880 and rs1050450; ***Single nucleotide polymorphism (SNP) analysis were carried out via Taqman assay and sanger sequencing respectively on rs17553719 and rs61816761).
Stage 2
2.1 Evaluation of the anti-ageing efficacy of a cosmetic treatment in the selected subjects: The aim of this study stage was to evaluate the anti-wrinkles, smoothing, moisturizing, firming, skin tone improvement and barrier protection efficacy of a cosmetic treatment. For that, 4 products were carefully developed in order to create a morning and night routine (Table 1):
| Day serum | Day cream | Night cream | Night serum | |
|---|---|---|---|---|
| Mode of action | -Anti-oxidant protection to help the skin fight free radicals throughout the day -Supportive function for day cream + sun screen booster |
-Daily protection with sunscreens -Skin hydrating formulation |
-Stimulate endogenous recovery processes -Works on firming the skin - Stimulate collagen synthesis and slow down breakdown |
-Focuses on hydration and loss of moisture -Barrier repairing |
| Formulation | Light o/w emulsion-easily absorbed | o/w emulsion-thicker texture, 20% fat | Light texture-non occlusive | Light gel-like structure |
| Basis | Broad spectrum anti- oxidants | Lipids | Collagen stimulating actives in a penetration stimulating basis | Mix of humectants (amino acids, urea, glycerine) |
| Key active ingredients | -SOD -Glutathion |
UVA and UVB filter, emollients | -Tripeptide-10 citrulline, palmitoyl tetrapeptide-3/5, palmitoyl -Tetrapeptide-7 |
-Skin identical lipids such as ceramides, phospholipids, -Cholesterol |
Table 1: Summary of the cosmetic products key attributes used in the scope of the clinical study.
• Day Serum: This formulation is enriched with antioxidants such as superoxide dismutase and glutathione, one of the front line defence mechanisms against free- radical injury, has Resveratrol at 0.10% which is efficient in increasing skin hydration, Empetrum Night Fruit Juice at 1.00% that boost skin tone and skin tension and also increases the microcirculation of the skin, a combination of SimmondsiaChinensis Seed Oil at 0.77%, Squalane at 0.17% and Solanum Lycopersicum Fruit Extract at 0.06% that has an anti-aging and a photo aging effect as well as skin whitening effect and VitisVinifera Juice Extract at 0.003% which helps to reduce wrinkles.
• Day Cream: This formulation in combination with the day serum supports the protection against free radicals from UV rays with the help of UVA and UVB filters (Polysilicone-15 at 5%, EthylhexylMethoxycinnamate at 4.99%, EthylhexylTriazone ate 3%, Methylene Bis- BenzotriazolylTetramethylbutylphenol at 1.5%) to protect the skin from the UV damage. In addition, it improves the skin condition by exerting a hydrating effect on the skin thanks to a combination of Glycerine at 1.4%, Phospholipids at 0.56%, Squalane at 0.2%, Safflower Oil and Palm Oil Aminopropanediol Esters at 0.19%, Sodium Hyaluronate at 0.08% and Ceramide-2 at 0.05%. This O/W emulsion containing 20% fats also contributes to the reduction of the transepidermal water loss (TEWL), and therefore to a stronger skin barrier. The product contains also emollients with antioxidant benefits (PrunusArmeniaca Kernel Oil at 2% and Macadamia Integrifolia Seed Oil at 0.68%) and calcium ketogluconate at 0.5% with benefits proven on skin elasticity.
• Night Cream: This formulation aims to act on wrinkles, skin smoothness and firmness due to a combination of different ingredients: MPCTM–Milk Peptide Complex (Whey protein) at 0.5%, PhytosanTM K (Glycerin, Glycine Soja (Soybean) Seed Extract) at 5%, Luteolin 98 MM (Citrus Reticulata (Tangerine) Peel Extract) at 0.5%, Phytocytol AGE (Polyglycerin-3, Butylene Glycol, Pentylene Glycol, Methylpropanediol, PuerariaLobata Root Extract, Chlorogenic Acids) at 10%, Pro-renew (Lactococcus Ferment Lysate) at 3%, Matrixyl 3000 (Glycerin, Butylene Glycol, Palmitoyl Tripeptide-1 and Palmitoyl Tetrapeptide-7) at 3% and Trylagen (Pseudoalteromonas Ferment Extract, Hydrolyzed Wheat Protein, Hydrolysed Soy Protein, Tripeptide-10 Citrulline, Tripeptide- 1, Lecithin, Butylene Glycol) at 5%. These ingredients have proven effects on thestimulation of the collagen synthesis, the increase of skin firmness and smoothness, besides presenting an anti-MMP1 and anti- MMP3 effect as well as inhibiting the formation of inflammatory Advanced Glycation End Products.
• Night Serum: This formulation presents a long-lasting hydration and skin barrier improvement effects, due to the ingredients DayMoist CLR ™ (Hydrolyzed corn starch and Beta Vulgaris (Beet) Root Extract) at 5%, Hygroplex HHG (Hexylene Glycol, Fructose, Glucose, Sucrose, Urea, Dextrin, Alanine, Glutamic Acid, Aspartic Acid, Hexyl Nicotinate, Hexylene Glycol, Fructose, Glucose, Sucrose, Urea, Dextrin, Alanine, Glutamic Acid, Aspartic Acid, Hexyl Nicotinate) at 5%, Skin’ential™ CS (Potassium Cholesterol Sulfate) at 0.3%, Skin’ential™ HA (Acetyl Glucosamine) at 2%, Vegeluron® Gel (Propanediol, TremellaFuciformis (Mushroom) Extract, Gluconolactone) at 3% and Glycoderm (Honey, Phospholipids, Sphingolipids, Hyaluronic Acid) at 5%.
2.2 Evaluation of the reduction of the skin erythema induced by UV light (antioxidant efficacy): The aim of this study stage was to evaluate in vivo the anti-oxidant efficacy of the day serum through the assessment of the minimum erythema dose (MED) produced 24 and 48 hours after UV irradiation in relation to a comparator product.
In order to perform this study stage, 14 female subjects with a mean age of 56.08 years old selected accordingly to the same DNA analysis performed before and respecting all the inclusion and exclusion criteria referred above were enrolled.
Erythema resulting from UV radiation exposure (after the trigger of the inflammatory process caused by ROS activation) appears approximately after 2 h of exposure and reaches a maximum after 24 hours. Usually starts to fade away after 48 hours [17]. To determine the anti- oxidant efficacy of the product, incremental series of delayed erythemal responses were induced on a number of small sub-sites on the skin and visually assessed and compared 24 h to 48 h after UV radiation for the treated and untreated test sites.
To perform this test, the skin color of the test area (back) of each subject was assessed with equipment Colorimeter® CL400 (Courage+Khazaka electronic GmbH, Germany) at Day 0 (baseline) in order to determine the ITAº (Individual Typology Angle) of each subject, and to adjust the adequate UV doses (MED) to each subject. For each subject, 3 test sites were needed: 1 test site for provisional MEDu determination, 1 test site for the investigational product application and 1 untreated test site used as control. In this stage only the Day Serum was tested. The region between the scapula line and the waist was the chosen anatomical region for the test area and the positions of the different test sites was distributed randomly on the back of subjects over the whole test. Each subject is exposed to 6 different UV doses per test site at baseline and after 5 product applications. 2 mg/cm2 of the Day Serum was weighed and applied in the respective skin test site. Five applications were performed before the UV exposure, with a minimum of 6 hours interval between eachapplication. The test area where the provisional MEDu was determined and where the investigational product was applied was randomized.
The equipment used to perform the irradiation was the Single port Solar UV Simulator Model 16S-150-001 (Solarlight®, United States of America) serial number #20300. Single port 16S-150- 001 has a 150 watt Xenon arc lamp with a continuous spectrum ranging from 290 to 400 nm. To ensure uniformity in spectral shape in SPF testing, the UV solar simulator uses a xenon arc lamp, filtered with a dichroic UV filter to minimize IR radiation, and UV shaping filters such as UG11/1 mm. A suitable warm-up time (10 min) was allowed for the UV solar simulator to stabilize before starting exposures, this is to ensure a consistent irradiance over the whole exposure period.
For each subject, at baseline an untreated test site was exposed to UV-light regarding the approximate value of the likely MEDu of each subject with a certain ITAº value of the skin to determine the minimal erythemal dose (MED) 20 ± 4 hours after UV exposure. The visually determined MED corresponds to the lowest UV dose that produces the first perceptible unambiguous erythema with defined borders appearing over most of the field (in at least 75%) of UV exposure, 20 ± 4 hours after UV exposure. After 5 product applications, both treated and untreated areas were exposed to specific doses of UV-light on each test sub- site: 0.5; 1.0; 1.25; 1.5; 1.75 MED. 24 h and 48 h after both treated and untreated skin areas being equivalently exposed to UV irradiation, the site with better erythema is visually classified as “ better ” or, in case of equal erythema, marked as “same” for each UV dose applied.
The evaluation was performed with the subjects in prone position and under the same lightening conditions in a room with matt, neutral wall colour, with about 653 lux, which are the recommended conditions for visual assessment.
All study procedures (products application, UV exposures and MED assessment) were carried out in the same controlled conditions of temperature and relative humidity.
A 10-minute acclimatization period of the exposed area of the subject was respected when the skin colour was evaluated (at the ITAº determination) and when the erythema was evaluated.
For the skin erythema results statistical analysis was performed at each time-point of evaluation (24 hours and 48 hours of the UV exposure). In order to assess if the distribution of the results was homogenous, non-parametric Chi-square test was applied. The significance value was established at 0.10 and a power of 0.90.
The entire study was performed in accordance with the Declaration of Helsinki Principles, including Ethics Committee approval by the Ethics Committee of inovapotek, Pharmaceutical Research and Development and also according to the principles of good clinical practices ICH-GCP. All participants gave written informed consent.
Evaluation of the anti-ageing efficacy of a cosmetic treatment
Results are shown in Tables 2-5 and Figures 3-5.
| Wrinkles’ depth (µm) | Skin roughness (µm) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Investigational treatment | Comparator treatment | Investigational treatment | Comparator treatment | |||||||||
| D0 (n=24) | D28 (n=24) | D56 (n=24) | D0 (n=24) | D28 (n=24) | D56 (n=24) | D0 (n=24) | D28 (n=24) | D56 (n=24) | D0 (n=24) | D28 (n=24) | D56 (n=24) | |
| Mean results | 113.38 | 105.83 | 104.79 | 105.79 | 101.13 | 106.92 | 28.21 | 25.57 | 25.45 | 24.93 | 23.93 | 24.18 |
| ± SD | 39.87 | 44.43 | 38.32 | 38.76 | 40.64 | 45.1 | 10.06 | 7.07 | 7.68 | 6.39 | 5.77 | 6.17 |
| Mean differences | _ | -11.36 (n=22) | -10.7 (n=23) | _ | -2.7 (n=23) | -2.26 (n=23) | _ | -1.62 (n=23) | -1.95 (n=23) | _ | -1 (n=24) | -0.27 (n=23) |
| ± SD | _ | 13.31 | 14.12 | _ | 18.34 | 16.79 | _ | 3.6 | 2.56 | _ | 2.85 | 2.99 |
| Maximum result (%) | _ | -35.92% | -30.43% | _ | -33.62% | -32.35% | _ | -26.85% | -29.72% | _ | -25.59% | -26.69% |
| p value (Before vs After) | _ | 0.024** | 0.023* | _ | _ | _ | _ | 0.024** | 0.001** | _ | _ | _ |
| p value (group vs group) | _ | 0.108** | 0.021* | _ | 0.108** | 0.021* | _ | 0.490* | 0.027* | _ | 0.490* | 0.027* |
*Paired T-Student test, **Wilcoxon test
Table 2: Overall results regarding the anti-aging evaluated parameters.
| Skin hydration (A.U.) | Skin TEWL (g/m2/h) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Investigational treatment | Comparator treatment | Investigational treatment | Comparator treatment | |||||||||
| D0 (n=24) | D28 (n=24) | D56 (n=24) | D0 (n=24) | D28 (n=24) | D56 (n=24) | D0 (n=24) | D28 (n=24) | D56 (n=24) | D0 (n=24) | D28 (n=24) | D56 (n=24) | |
| Mean results | 36.16 | 44 | 44.61 | 36.11 | 43.88 | 43.9 | 6.93 | 7.45 | 6.45 | 6.75 | 7.38 | 6.56 |
| ± SD | 6.61 | 4.52 | 6.75 | 6.1 | 4.65 | 6.18 | 1.46 | 1.58 | 1.54 | 1.39 | 1.49 | 1.52 |
| Mean differences | _ | 7.85 | 8.46 | _ | 7.77 | 7.79 | _ | 0.52 | -0.6 | _ | 0.63 | -0.19 |
| ± SD | _ | 4.44 | 6.73 | _ | 4.96 | 6.45 | _ | 1.77 | 1.57 | _ | 1.49 | 1.9 |
| Maximum result (%) | _ | 69.20% | 54.11% | _ | 67.66% | 59.07% | _ | -40.91% | -38.40% | _ | -27.78% | -44.44% |
| p value (Before vs After) | _ | <0.001* | <0.001* | _ | _ | _ | _ | 0.167* | 0.172* | _ | _ | _ |
| p value (group vs group) | _ | 0.938* | 0.514* | _ | 0.938* | 0.514* | _ | 0.762* | 0.349* | _ | 0.762* | 0.349* |
*Paired T-Student test, **Wilcoxon test
Table 3: Overall results regarding the anti-aging evaluated parameters.
| R0 – Uf (mm) | R2 - Ua/Uf (mm) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Investigational treatment | Comparator treatment | Investigational treatment | Comparator treatment | |||||||||
| D0 (n=24) | D28 (n=24) | D56 (n=24) | D0 (n=24) | D28 (n=24) | D56 (n=24) | D0 (n=24) | D28 (n=24) | D56 (n=24) | D0 (n=24) | D28 (n=24) | D56 (n=24) | |
| Mean results | 0.23 | 0.215 | 0.197 | 0.222 | 0.216 | 0.202 | 0.519 | 0.526 | 0.469 | 0.505 | 0.499 | 0.481 |
| ± SD | 0.028 | 0.033 | 0.022 | 0.035 | 0.035 | 0.027 | 0.051 | 0.065 | 0.067 | 0.085 | 0.077 | 0.055 |
| Mean differences | - | -0.014 | -0.033 | - | -0.007 | -0.02 | - | 0.007 | -0.05 | - | -0.005 | -0.023 |
| ± SD | - | 0.036 | 0.033 | - | 0.037 | 0.029 | - | 0.049 | 0.057 | - | 0.07 | 0.075 |
| Maximum result (%) | - | -0.3111 | -0.3333 | - | -0.2653 | -0.2682 | - | 0.2395 | 0.0611 | - | 0.323 | 0.2912 |
| p value (Before vs After) | 0.061* | <0.001* | - | - | - | - | 0.491* | <0.001* | - | - | - | |
| p value (group vs group) | - | 0.537* | 0.174* | - | 0.537* | 0.174* | - | 0.548* | 0.206* | - | 0.548* | 0.206* |
*Paired T-Student test, **Wilcoxon test
Table 4: Overall results regarding the anti-aging evaluated parameters.
| R7- Ur/Uf (mm) | ||||||
|---|---|---|---|---|---|---|
| Investigational treatment | Comparator treatment | |||||
| D0 (n=24) | D28 (n=24) | D56 (n=24) | D0 (n=24) | D28 (n=24) | D56 (n=24) | |
| Mean results | 0.24 | 0.26 | 0.235 | 0.237 | 0.242 | 0.241 |
| ± SD | 0.021 | 0.036 | 0.038 | 0.041 | 0.04 | 0.03 |
| Mean differences | - | 0.02 | -0.005 | - | 0.005 | 0.004 |
| ± SD | - | 0.031 | 0.034 | - | 0.026 | 0.036 |
| Maximum result (%) | - | 0.4382 | 0.2144 | - | 0.3339 | 0.4715 |
| p value (Before vs After) | - | 0.004* | 0.485* | - | - | - |
| p value (group vs group) | - | 0.123* | 0.450* | - | 0.123* | 0.450* |
*Paired T-Student test, **Wilcoxon test
Table 5: Overall results regarding the anti-aging evaluated parameters.
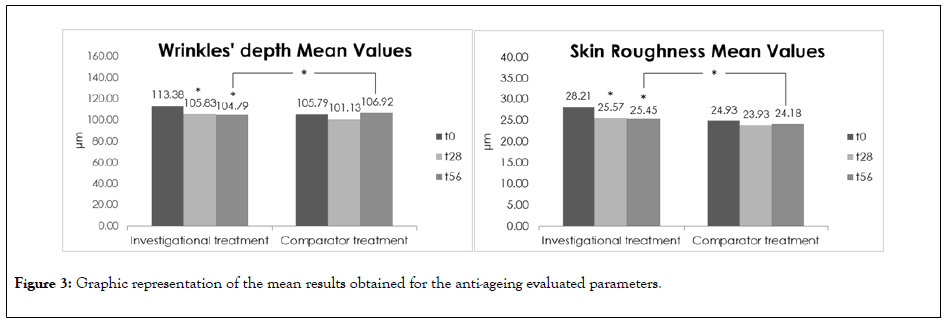
Figure 3: Graphic representation of the mean results obtained for the anti-ageing evaluated parameters.
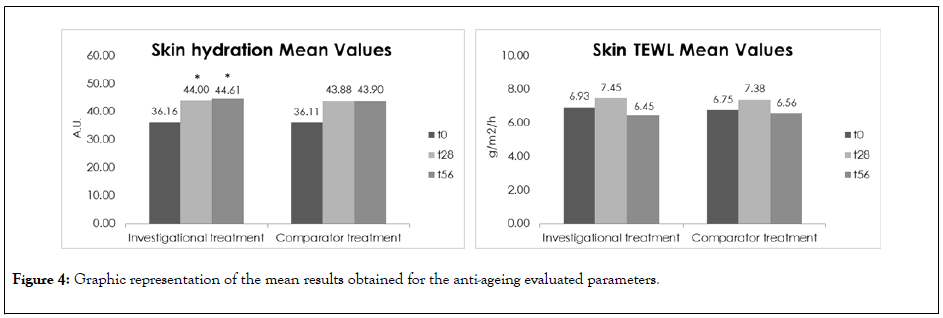
Figure 4: Graphic representation of the mean results obtained for the anti-ageing evaluated parameters.
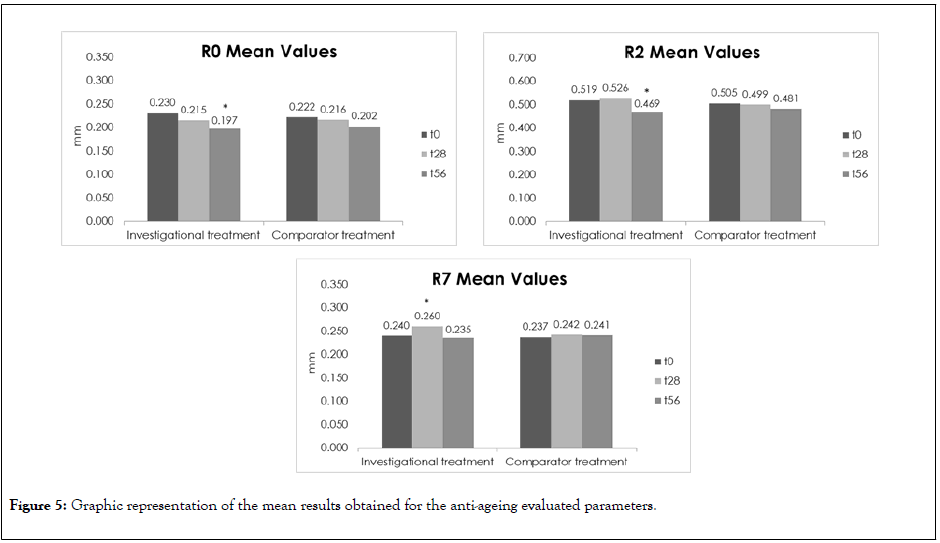
Figure 5: Graphic representation of the mean results obtained for the anti-ageing evaluated parameters.
After 28 days and 56 days of the investigational cosmetic treatment application, a statistically significant decrease up to -35.92% (D28) and -30.43% (D56) in wrinkles’ depth and up to -26.85% (D28) and -29.72% (D56), in skin roughness in the crow’s feet region was observed, evidencing that the treatment has an anti-wrinkles and smoothing effect. The investigational treatment has a statistically significant higher anti-wrinkles and smoothing effects than the comparator product 56 days after the treatment.
The subjective results are in accordance with these results as 91.67% (D28) and 95.83% (D56) of the subjects considered that the investigational cosmetic treatment has a slightly to strong anti-wrinkles effect and 100% (D28) and 95.83% (D56) of the subjects considered that the treatment has a slightly to strong smoothing effect.
For the investigational treatment a statistically significant hydrating effect up to 69.20% (D28) and 54.11% (D56) was observed after the treatment, respectively. However, no significant differences were found between the investigational treatment and comparator product as both treatments presented similar moisturizing effects at these time-points.
Regarding the TEWL results, both treatments presented a similar lack of effect of the skin barrier function improvement. However, it is relevant to mention that both treatments did not compromise the skin barrier function, maintaining its characteristics after the treatment.
For the total deformation of the skin (R0, Uf) significantly decreased up to - 33.33% (D56) for the investigational treatment meaning that the skin became more elastic but no statistical differences were found between the investigational and comparator products concerning this parameter. Nevertheless, the investigational treatment appears to present a higher efficacy concerning this parameter.
Regarding the skin firmness (R7, Ur/Uf), a significantly increase up to 43.82% (28 days) was observed and a parameter decrease at D56 was verified which was not statistically significant. The treatments’ comparison demonstrate that after 28 and 56 days of products ’ application, the two treatments present similar effect on the skin firmness.
Besides these results, the subjects opinion was positive with 83.33% and 87.5% of the subjects considered that the skin is slightly to intensively with a more homogeneous tone 28 and 56 days of the investigational cosmetic treatment, respectively.
Overall, subjects perceived a better efficacy, for the investigational product than the comparator product.
The positive results regarding anti-ageing, namely regarding the anti-wrinkles and smoothing efficacy effects, highlights a possible correlation between a ‘high risk profile’ on collagen breakdown that these subjects present and a skin predisposition for the treatments that prevent the collagen failure.
The observed increase on skin hydration had no statistical significance versus the control group and the lack of results regarding the TEWL was also expected since the subjects had a ‘low risk profile’ when it comes to dehydration and impaired barrier function.
The skin wasinherently well hydrated and the skin barrier was believed to be intact, based on the genetic information and questionnaire that we implemented. The treatment helps to keep the barrier intact and is not interfering with the natural barrier function.
Regarding the second part of the stage 2 of the study for the evaluation of the antioxidant effect of a cosmetic product, after 5 product applications, both treated and untreated areas were exposed to specific doses of UV-light on each test sub-site: 0.5; 1.0 and 1.5 MED. 24 h and 48 h after both treated and untreated skin areas being equivalently exposed to UV irradiation, the site with better erythema is visually classified as “better” or, in case of equal erythema, marked as “same” for each dose.
Results are expressed on Table 4 and Figure 6A, and the classification given was regarding the product test when compared to the untreated site.
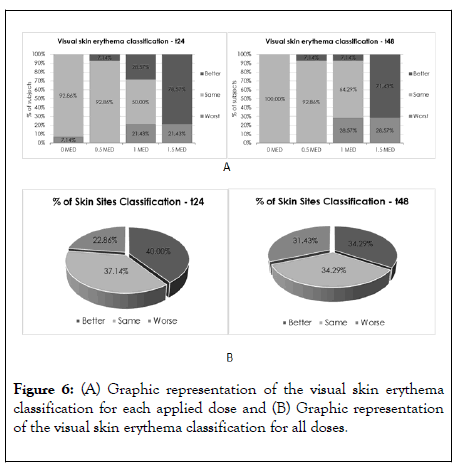
Figure 6: (A) Graphic representation of the visual skin erythema classification for each applied dose and (B) Graphic representation of the visual skin erythema classification for all doses.
The mean values represent the mean punctuation given (between -1, 0 and1) and higher the mean value, closer the classification to the “better” option.
When observing the lower MED doses applied (0 and 0.5 MED), as expected, the visual skin erythema classification distribution was unequal prevailing the “same” classification, once the erythemal response is practically inexistent in both test sites.
In this case, the 0 MED and the 0.5 MED are doses which do not deliver any erythemal responses as they are doses below the minimum erythemal dose that is capable of provoking a visible erythema.
Thus, no differences were observed between the treated and untreated area, with 92.86% to 100% of the subjects presenting the classification “ same ” regarding visual skin erythema evaluation after 24 hours and after 48 hours of UV irradiation.
A similar scenario was observed 48 hours after the UV irradiation for the 1.0 MED dose with 64.29% of the subjects presenting the classification “same” regarding the visual skin erythema evaluation. For this dose, 24 h after UV irradiation, 28.57% of the subjects showed a “better” erythema evaluation, but the results were not enough for statistical difference.
For the 1.5 MED dose 24 hours after the UV irradiation statistically significant differences were observed between the treated and untreated area, as 78.57% of the subjects presented a “better” visual skin erythema evaluation. After 48 hours nearly statistically significant differences were observed (p=0.109), as 71.43% of the subjects presented a “better” visual skin erythema evaluation, indicating an anti-oxidant efficacy of the investigational product for this UV dose at all time-points.
When analysing the erythema classifications for all the UV doses applied as a whole (Figure 6B), 40% of the skin sites exposed presented a “better” classification in comparison with the untreated area after 24 h of the UV irradiation.
For the time-point 48 h, 34.29%, of the skin sites exposed presented a “ better ” classification in comparison with the untreated area. However, it was not statistically significant for both time-points.
The antioxidant response might be explained by the ‘medium risk profile ’ assigned to this antioxidant cluster. SNPs were present in 1 allele and 1 copy was probably functional. At lower doses the day serum did not show a positive effect as the endogenous antioxidants were possibly effective enough to deal with the weak UV doses applied.
24 h after the UV irradiation the erythemal reactions were, as expected, more pronounced which led to more conclusive results on the product efficacy at this time-point.
With higher doses, the natural endogenous antioxidant capacity was compromised and overwhelmed by the free radicals and the day serum delivered an additional significant support at initial time-points (24 hours).
At advanced time-points (48 hours) the significance of the better results fades away. A possible explanation might be that the endogenous antioxidants triggered by the UV doses on the untreated site start to take action contrarily to what happened on the treated site which had no more product supply and might be suffering from a late production of endogenous antioxidants due to previous product protection.
Generally, individuals rely on any information about their skin condition in order to make a decision about the type of products (e.g., skin care, cosmetic, etc.) needed for their skin. Typically, consumers choose the desired products according to their limited understanding of their skin quality and often from a self-assessment. However, it is important the consumer understands the breach between their current skin condition versus its genetic potential or the genetic predisposition of their skin for the risk of developing skin imperfections [18]. Experts have long recognised a list of active ingredients that play a key role in skin health. A possible direct-response relationship between an active ingredient and its target allows the ingredient to provide the best effect of a product [19].
The results obtained in this study showed that a skin care regimen designed to address the specific needs of a genetic risk profile characterized by high risk for collagen breakdown and medium risk for low anti-oxidant production was effective on decreasing wrinkles, improving skin roughness and protecting the skin from UV oxidative damage.
These results highlight the interest of analysing an individual's genetic material, raising the possibility of providing a personalized treatment for that individual. Nevertheless, further studies should be designed in order to confirm the potential observed in this investigation whether to include a higher sample size whether to include two groups of subjects, with different responsive genotypes, studying different polymorphisms and substantiating the notions presented with this study.
Citation: Geusens B, Rodrigues L, Matias R, Tavares B, Fonesca AL, Silva AM, et al. (2020) A Double Blinded, Randomized, Controlled Split-Face Study to Investigate the Efficacy of a Tailor-made Anti-Ageing Skin Care Regimen Adapted to a Genetic Skin Ageing Risk Profile. J Clin Exp Dermatol Res. 11: 527. DOI: 10.35248/2155-9554.20.11.527
Received: 06-Jul-2020 Accepted: 20-Jul-2020 Published: 27-Jul-2020 , DOI: 10.35248/2155-9554.20.11.527
Copyright: © 2020 Geusens B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.