
Journal of Clinical Trials
Open Access
ISSN: 2167-0870

ISSN: 2167-0870
Research Article - (2021)Volume 11, Issue 4
Background: CKD is a common public health problem affecting over 200 million people worldwide. The United States accounted for about 30 million CKD patients with high morbidity, mortality and enormous health costs. Despite advancement in treatment options of risk factors for CKD like hypertension and diabetes, progression to end stage kidney disease and complications remain high. Observations of gut dysbiosis associated with progressive kidney disease, systemic inflammation and uremic toxin retention led to trial use of probiotics in restoring gut microbiome to ameliorate CKD. Studies have shown benefits in restoring gut microbiota with probiotics retarded progression, reduced uremic toxins, and improved quality of life in CKD. This clinical trial is to evaluate safety and efficacy of probiotic formulation US APR 2020 in patients with CKD stage 4 with an aim of retarding progression of CKD to End Stage Kidney Disease (ESRD).
Methods: All eligible adult patients with CKD (eGFR 15-29 mls/min) with serum creatinine >2.5 mg/dl will be randomly assigned to either US APR 2020 (Group A) or Placebo (group B). The participants will be followed up monthly from baseline to 6 months of end of treatment with schedule of assessments at each visit. The primary end points will be based on <10% of adverse events and mean reduction of 40% decline in eGFR between the two groups at end of treatment.
Discussion: This trial protocol outlined the design to evaluate Phase 2 safety and efficacy of FDA approved probiotic IND for treatment of patients in CKD stage 4 with primary objective of retarding renal disease progression. Data from this trial will inform planning for a Phase 3 clinical trial as part of unique and innovative drug development program for these categories of patients.
Chronic kidney disease; Probiotics; Clinical trial
CKD: Chronic Kidney Disease; CVD: Cardiovascular Disease; ESRD: End Stage Renal Disease; BMI: Body Mass Index; SF 36 QOL: Short Form 36 items Quality of Life; IND: Investigative New Drug; FDA/CBER: Food and Drug Administration/Center for Biologics Evaluation and Research; MedDRA: Medical Dictionary of Regulatory Affairs; TEAE: Treatment Emergent of Adverse Events; PP: Per Protocol; ITT: Intent To Treat; ANCOVA: Analysis of Covariance; AE: Adverse Events.
Chronic kidney disease is a common public health problem that affects about 10% of adults worldwide and it is estimated that 30 million adults in the United States are affected with CKD and most of them remaining undiagnosed [1]. It is the ninth leading cause of death associated with enormous socio-economic costs to individuals and health systems. In the United States, Medicare spending on treating patients with CKD estimated to be $ 79 billion, with end stage kidney disease care gulping about $ 35 billion [1,2]. The important traditional risk factors for CKD in adults include diabetes mellitus and hypertension with other contributory factors like heart disease, obesity, family history of CKD, history of kidney damage and older age groups. CKD progresses slowly and sometimes asymptomatically to End Stage Renal Disease (ESRD) and often develop Cardiovascular Disease (CVD) as the main leading cause of morbidity and mortality in CKD [3,4].
The gut microflora of CKD patients differs considerably from normal healthy subjects with a distinctly altered bacterial composition or dysbiosis [4]. This dysbiotic gut microflora has been shown to increase uremic and bacterial toxins in CKD patients [5]. It has been proposed that toxins generated by gastrointestinal dysbiosis and disseminated into systemic circulation through the small and large bowel, may contribute to CKD pathogenesis by inducing metabolic imbalance and several immunological processes [4,5].
CKD is associated with dysbiosis of intestinal microbiota, retention of uremic toxins, increased proinflammatory cytokine and acute phase reactants production [6,7]. Studies have shown these pathophysiological processes correlate with renal inflammatory changes, oxidative stress, glomerular and tubule-interstitial injury, and fibrosis. These pathogenetic mechanism manifests as proteinuria and decline in GFR which clinically correlates with progressive deterioration of renal function [4-7].
Non-clinical studies in animal models of CKD demonstrated that probiotics administration enhanced survival, body weight gain and slowed kidney injury progression in rat and feline models [8,9] and similar observations were seen in improvement of glomerular filtration rate in the dog model of CKD [10]. Other studies showed that administration of probiotics with uricolytic bacteria reduced uric acid levels and high blood pressure in hyperuricemia animal models that could potentially have clinical benefits in hypertension and kidney disease [7,11].
Administration of probiotics modulates host immunity at intestinal and systemic levels through microbial metabolism of intestinal dietary proteins and complex carbohydrates leading to amelioration and slowing progression of kidney injury [4,11]. Previous human studies have demonstrated the beneficial effects of probiotics on inflammation, uremic toxins, and quality of life among CKD patients, however, its usefulness in preservation of GFR is yet to be confirmed in a high-quality intervention trial [12-14]. Previous studies and Real-World Experience (RWE) of Kibow Biotech probiotic proprietary health supplement product in a sample of CKD patients were found to be safe, reduced uremic toxins (creatinine, BUN, p-cresol sulfate, Indoxyl sulfate, Uric Acid), inflammatory markers (CRP), improved hematological indices and quality of life [15,16].
Studies in various stages of CKD patients have shown safety and clinical benefits of probiotics formulation with improvements in GFR, metabolic profiles and quality of life assessments [17,18]. The link between gastrointestinal tract, intestinal microbial flora and kidney dysfunction (Gut-Kidney axis) has become a potential therapeutic target for CKD [19,20].
Consensus is that newer medications modulating the gut microbiome using probiotics are needed through a drug development pathway of clinical trial to prove safety and efficacy in slowing or retarding CKD progression and improving quality of life [21]. From these observed benefits of probiotics in late stages of CKD in delaying progression and improving quality of life, it is expected that the outcome of this clinical trial could potentially delay initiation of dialysis, reduce healthcare costs and improve quality of life with additional secondary benefits of reducing cardiovascular disease complications [4,21].
The USA Food and Drug Administration (US-FDA) has approved US APR 2020 as an Investigational New Drug (IND) for Kibow Biotech for a double blind, placebo-controlled Phase 2b clinical trial to evaluate the safety and efficacy in patients with chronic kidney disease stage 4. The US APR 2020 is a live biotherapeutic product containing a mix of Streptococcus thermophilus KB19, Lactobacillus acidophilus KB27 and Bifidobacterium longum KB31. US APR 2020 microbes are naturally occurring, genetically not modified, and approved for human consumption and Generally Regarded as Safe (GRAS) under US FDA guidelines.
The primary objective of this clinical trial is to evaluate safety and clinical efficacy of US APR 2020 and secondary objectives are to evaluate effect of US APR 2020 on changes in basic blood uremic, metabolic, hematologic, inflammatory markers and quality of life in patients with CKD stage 4.
The design, conduct and reporting of this study will follow the protocol, International Conference on Harmonization/Good Clinical Practice (ICH/GCP) guidelines, and all appropriate regulatory requirements of participating institutions. All Investigators participating in this study will have documented training in GCP. Independent monitoring of the trial will be accomplished utilizing a Data Safety Management Board (DSMB) and clinical trial management will be outsourced to a Contract Research Organization (CRO).
Study design
This is a double-blind, randomized, placebo-controlled Phase 2 clinical trial to evaluate safety and efficacy of US APR 2020 in subjects with chronic kidney disease stage 4. This will be a multicenter study of 20 USA sites involving 630 patients with 315 randomized to each arm of the study. The patients will be randomized in a 1:1 ratio to intervention arm (US APR 2020 administered orally 2 capsules of 90 billion CFU per day of Live Biotherapeutic product) and placebo (2 capsules). All enrolled patients will continue with standard of care as determined by their Physician/Nephrologist. The duration of study conduct will be 6 months consisting of 6 visits upon enrollment in Figure 1.
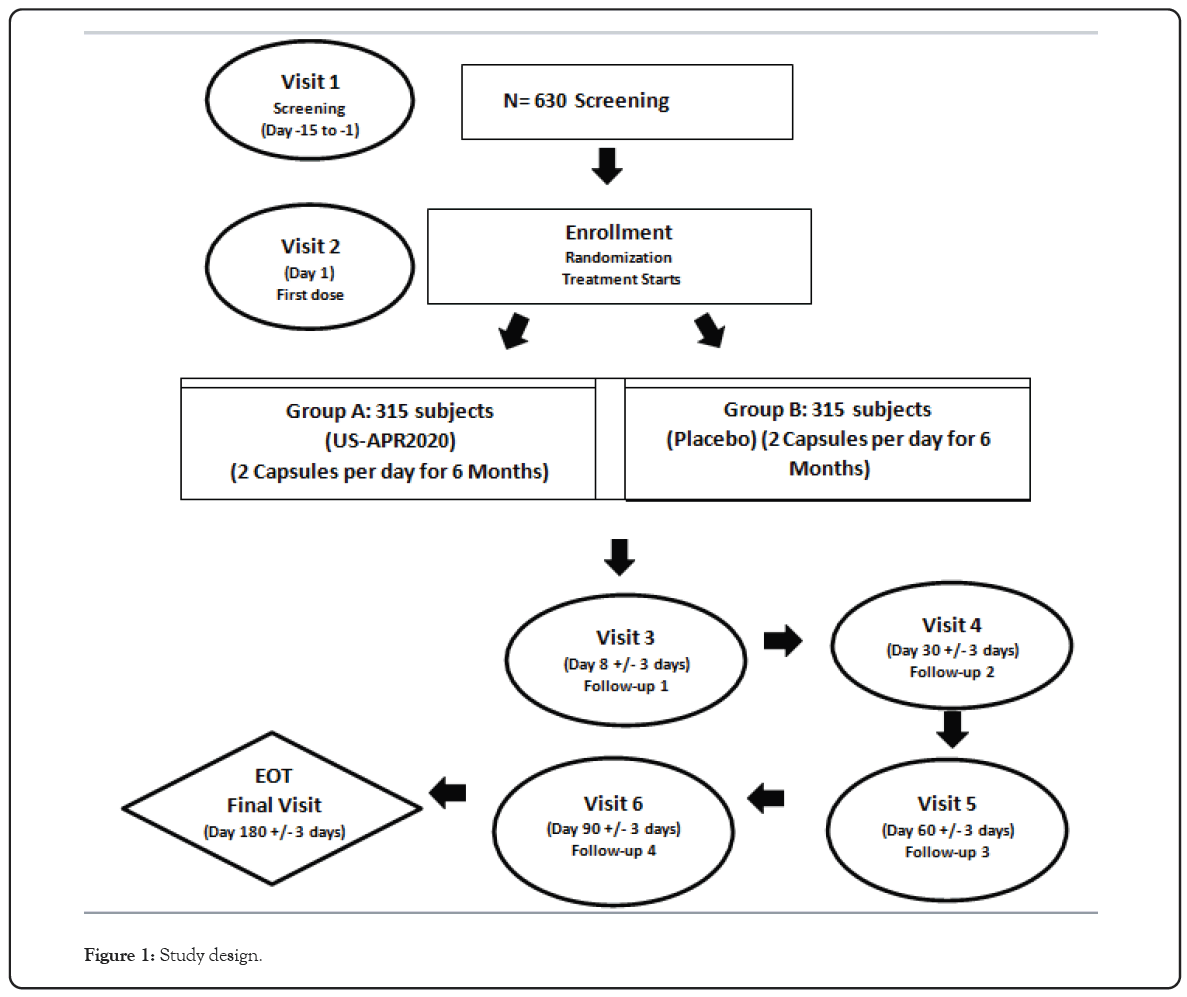
Figure 1: Study design.
Study population
All patients will be recruited from Nephrology clinics and practices of 20 USA sites through Principal Investigators (PI) who will have clinical trial agreement and collaborations with Kibow Biotech Inc.,
The inclusion and exclusion criteria for enrollment eligibility in the study will be as follows
Inclusion criteria: 1. Adults between ages of 18 to 80 years.
2. CKD stage 4 (eGFR 15-29 mls/min) with declining kidney function for a period of more than 6 months.
3. Serum Creatinine >2.5 mg/dl.
4. Adherence to Low Protein Diet (LPD) of 0.6-0.8 g/kg/day.
Exclusion criteria: 1. Those on probiotics in the past 3 months.
2. Pregnancy, breast feeding or females of childbearing potential who are unwilling to use reliable form of contraception.
3. Immunosuppressant medications therapy specific to immune mediated renal disease.
4. HIV/AIDS.
5. Underweight (BMI ≤ 18.5 kg/m2)
6. Subjects with infection requiring oral antibiotics.
7. Those with gastrointestinal disease (irritable bowel syndrome, anal fissures, anal fistula, perianal abscess/infections, diverticular disease, colitis, and colon polyps).
8. Those with internal prosthesis of any kind.
9. Those on anticoagulant therapy
10. Those on peritoneal therapy.
11. Those with acute kidney injury.
12. Those with mental conditions or medically debilitating disease/disorder other than CKD, which in the judgement of the investigator would interfere with or contraindicate to adherence to study protocol or affect ability to give informed consent or overall prognosis of the patient.
If patients meet all inclusion and exclusion criteria they will be screened and enrolled into the study by the principal investigators at any of the approved designated clinical trial sites. The clinical trial management will be carried out on behalf of Kibow Biotech through a Clinical Research Organization (CRO).
Following screening and enrollment, eligible patients will be randomized to either Group A (US APR 2020) or Group B (Placebo). Thereafter the visits for schedule of assessments will be done monthly for 6 months which is end of treatment. All patients who completed the study will be followed for another 6 months for safety assessment through an open label study.
The study schedules
Screening phase: This is designed to determine whether subjects will be eligible to proceed to treatment phase with schedule assessment which consist of obtaining written informed consent from subjects (or Legally Acceptable Representatives) and Health Insurance Accountability Act (HIPAA) authorization prior to study related procedures. The following evaluations and examination will be done prior to clinical trial at screening and subsequent visits as outlined in the study design (Figure 1).
• Signed informed consent
• Eligibility criteria review
• Demographic and medica history review
• Physical exam and vital signs
• Pregnancy tests
• General blood chemistry tests
• General hematology tests
• Blood inflammatory and nutritional markers
• Kidney Injury markers tests
• Liver function tests
• Modified SF36 QOL questionnaire
• HIV test
• Concomitant medication review
All screening information will be documented in subjects source document. For consented subjects who do not meet eligibility criteria at screening will have electronic Case Reporting Form (eCRF) completed as screen failure. The eligible consented subjects will have their information transcribed onto the appropriate section of eCRF and which will be part of baseline criteria. All the clinical trial documentation, data entry and Trial Master File (eTMF) will be in electronic format.
Treatment phase
Subjects who successfully complete the screening phase will be enrolled and randomized to US APR2020 or Placebo in a 1:1 ratio to one of the treatments groups; Group A: US APR 2020 2 capsules per day for 6 months and Group B Placebo 2 capsules per day for 6 months. Subjects will be monitored by clinical staff for 30 mins after ingestion of first dose for features of allergic reaction. During first visit of treatment phase assessment to be performed include medical history, physical exam and vital signs and all tests done at screening phase. Fecal sample for microbiome analysis will be collected. Investigative study product dispensation and count review, adverse event review and concomitant medication review will be carried out.
Follow-up phases
To assess safety and tolerability, follow up monitoring visits will be carried out at week 1 after initial dose and subsequently on days 30, 60, 90, 120, 160 (± 8 days) post initiation of first dose. At each visit following assessment to be performed include medical review, all previous laboratory tests, Modified SF 36 QOL questionnaire, study product count review and concomitant medical review.
Final visit/End of treatment
The final visit will be conducted on day 180 and is designed to determine subject’s response to US APR 2020 or Placebo treatment. The assessments at final visit include medical review, physical exam, all previous laboratory tests done at baseline, fecal microbiome stool analysis, modified SF 36 QOL questionnaire, study product count review, adverse event review and concomitant medication review.
Laboratory tests
All blood work for chemistry panel, hematology, inflammatory markers, immunological markers and kidney injury markers will be obtained at baseline and at each of the six scheduled visits with analysis done by a central laboratory.
To control for probable acute effects of intervention with probiotic- based IND on non-GFR determinants of serum creatinine, the eGFR threshold will be based on both serum creatinine and Cystatin C measurements and both eGFRscr and eGFRcys-c will be calculated at baseline, 3 months and end of treatment for all enrolled subjects.
For Kidney injury markers and gut generated uremic toxins analysis will be done in the research laboratory of Kibow Biotech. Serum samples for exploratory markers will be done in Kibow research laboratory using a Waters Ultra Performance Liquid Chromatography-Mass Spectrometry (UPLC-MS) instrument.
Fecal microbiome analysis will be done using 16s rRNA illumina sequencing to measure microbiome profile at genus level in both groups before and after treatment.
Renal ultrasound will be done for patients without a recent report in their health records.
All details of investigations and procedures are as outlined in the schedule of assessment in Table 1.
| Procedures | Visit 1. Day -15 to -1 Screening |
Visit 2*. Day 1 Enrollment Randomization on Treatment |
Visit 3. Day 8 ± 3 days Follow-up 1 Month 1 |
Visit 4. Day 38 ± 3 days Follow- up 2 Month 2 |
Visit 5. Day 68 ± 3 days Follow- up 3 Month 3 |
Visit 6 to Visit 7 | EOT Day 180 ± 3 days Final Visit Month 6 |
|---|---|---|---|---|---|---|---|
| Informed Consent Form (ICF) | X | ||||||
| Inclusion/exclusion criteria review | X | ||||||
| Demographic review (1) | X | ||||||
| Medical history review (2) | X | X | X | X | X | X | |
| Physical exam and vital signs (3) | X | X | X | X | X | X | |
| Pregnancy test | X | X | X | X | X | X | |
| Blood uremic metabolic markers (4) | X | X | X | X | X | X | |
| Blood cell counts (5) | X | X | X | X | X | X | |
| General hematology tests (6) | X | X | X | X | X | X | |
| Blood inflammation markers (7) | X | X | X | X | X | X | |
| Blood nutrition markers tests (Albumin and cholesterol) | X | X | X | X | X | X | |
| Kidney injury markers tests (8) | X | X | X | X | X | X | |
| Kidney morphology ultrasound (Pelvi-calyceal separation (PCS) analysis) |
X | ||||||
| General blood chemistry (9) | X | X | X | X | X | X | |
| Liver function tests (10) | X | X | X | X | X | X | |
| X | X | X | X | X | X | ||
| Modified SF36 QOL questionnaire (12) | X | X | X | X | X | X | |
| Study product dispensation (13) | X | X | |||||
| Study product count review | X | X | X | X | X | ||
| HIV test | X | X | |||||
| Adverse event review | X | X | X | X | X | ||
| Concomitant medication review (14) | X | X | X | X | X | X |
Note: 1) Date of birth, gender, race and ethnicity; 2) General health history such as illnesses other than CKD and bacterial infections; 3) Anthropometric parameters (Weight, Body Max Index (BMI), Waist Hip Ratio (WHR)), blood pressure, pulse rate, temperature, and physical symptoms; 4) Blood urea, blood urea nitrogen, uric acid, serum creatinine, Creatinine eGFR (estimated using the formula: 186 X (serum creatinine/88.4)-1.154 X (Age)-0.203 X (0.742 if female) X (1.210 if black)). Serum Cystatin C and eGFRcys at 0,3,6 months; 5) White Blood Cells (WBC); Red Blood Cells (RBC) and platelets; 6) Hemoglobin (Hgb), Hematocrit (Hct) and ferritin; 7) C-Reactive Protein (CRP) and Erythrocyte Sedimentation Rate (ESR); 8) Blood levels of Kidney Injury Molecule (Kim-1), Neutrophil Gelatinase-associated Lipocalin (NGAL), Indoxyl Sulfate (IS) and Trimethylamine-N-Oxide (TMAO); 9) Magnesium, calcium-phosphorus product, intact Parathyroid Hormone (PTH), bicarbonate, potassium, chloride, glucose, calcium, sodium and total protein; 10) Aspartate aminotransferase (AST/SGOT), alanine aminotransferase (ALT/SGPT) and Alkaline Phosphatase (ALP) longum; 12) As per Reference [22]; 13) 3 bottles will be provided (3-month supply); 14) Over the counter and prescription medicine, vitamins and/or herbal supplements.
Table 1: Schedule of assessments.
Outcome measures/End points
The primary end points are 1) Presence of adverse events in less than 10% of study population as a measure of safety 2) A mean reduction of 40% decline of eGFRcr as per NKF-US FDA guidelines from baseline to end of treatment in the group treated with US APR2020 as compared to placebo [22].
The secondary end points are 1) improvement in basic blood uremic and metabolic markers in US APR 200 group compared to placebo 2) improvement in hematological parameters in US APR 2020 group compared to placebo 3) Reduction in C reactive Proteins in US APR 2020 group compared to placebo 4) Percent change from baseline in rating scale (Modified SF 36 QOL questionnaire at 24 weeks in US APR 2020 group compared to placebo 5) progression of kidney failure (end stage or dialysis) [23].
The exploratory markers will be analyzed by comparing changes from baseline to end of treatment between US APR 2020 group and placebo of kidney injury markers (NGAL, KIM-1,) and gut derived uremic toxins (Indoxyl sulfate, P-cresyl sulfate Trimethylamine N-Oxide) and fecal microbiome profile. The Calcium, phosphate, Parathyroid hormone, blood electrolytes, liver function parameters, Anthropometric measurements will also be carried out.
Statistical analysis sample size consideration
Sample size calculation was based on assumption that the mean eGFR difference in active drug and placebo to be 1.0 ml/min/1.73 m2 (an expected change of 22 to 21.5 in treatment group vs. 22 to 20.5 in control group), a common standard deviation of 4 ml/ min/1.73 m2, Alpha level 0.05, powered at 80%, the study would require about 252 per group. Randomization ratio of 1:1 between treatment and placebo group and dropout rate of 20%, the sample size of 315 subjects per arm would be needed to show superiority of active treatment over placebo, making a total of 630 subjects for enrollment in the trial.
Assessment of safety
Adverse events will be coded using most recent version of Medical Dictionary of Regulatory Activities (MedDRA) [23,24]. All Treatment Emergent Adverse Events (TEAEs) will be recorded for events that occurred after first randomized treatment. Safety will be assessed based on following incident of TEAEs, incidence of withdrawal due to Adverse Events (AEs), change in laboratory values, Change in vital signs.
Assessment of efficacy
The primary analysis of end points will be conducted using Per Protocol (PP) population and to assess the consistency of the primary results supportive secondary analysis using Intention To Treat (ITT) population. Both analysis of primary and secondary end points will be based on end point that are continuous in nature using number of observations, mean, median, minimum, maximum and standard deviation values presented as descriptive summary. For inferential statistics, if normality assumption is met, t-Test or ANCOVA using baseline as covariate. If normality assumption not met, an ANCOVA analysis on rank transformed data or other non-parametric method will be used. For categorical end points, frequency counts and percentages will be presented as descriptive summary and Chi-square test, or Logit model will be used for inferential statistics.
This FDA approved probiotic based IND for a Phase 2b randomized controlled clinical trial for subjects with CKD stage 4 is probably one of the first approval for a live biotherapeutic product in the chronic renal disease therapeutic area. Probiotics is well established in health supplement market and food industry with some level of presence in digestive, oncology, heart and mental health therapeutic areas because of linkage between gut microbiome and these organ systems [4,21].
With increasing emphasis on patient centered care inform of patient voices and choices in management of chronic kidney disease, innovative therapy like USA APR 2020 is potentially for unmet needs of patients in CKD stage 4 who may want to delay or avoid dialysis and opting for a conservative management approach. This may be a breakthrough therapy for economically and technologically disadvantaged communities and countries with limited access to renal care, unavailable and unaffordable renal replacement therapy. Demonstrating a successful recruitment and trial conduct of this study in CKD stage 4 subjects will inform future larger Phase 3 clinical trial.
Practical concerns that may impact this trial are non-compliance with investigative product due to additional pill burden in participants who are on multiple medications due to co-morbidities. There could be significant drop out rate of subjects due to rapid decline in renal function in some participants. There may be challenges with scheduling of in-person follow up visits with the patient’s routine clinic visits might affect protocol schedule of assessment.
Despite these potential barriers, this Phase 2 clinical trial will provide the necessary data to inform planning for Phase 3 trial in further confirming safety and efficacy of probiotic products in management of patients in CKD stage 4 by delaying progression to ESRD.
Data sharing not applicable at this article as no datasets were generated or analyzed by this study.
Ethical approval for the trial was obtained from WCG IRB (# 20212330) and in addition to each site IRB. Written informed consent will be obtained from all participants. Data and safety monitoring of the trial will be done by an independent Data Safety Management Board (DSMB) who have no conflict of interest in trial outcome or affiliation with sponsor.
All authors made substantial contribution to the concept and design of the study, took part in drafting and revising the protocols and article to meet requirements of FDA, agreed to submission to the Journal. EA is the lead, Study design, Project Manager/ Clinical Trial coordinator, NR is Chief research scientist and IND developer, PR is Trial regulatory and clinical operations manager, UV is laboratory research scientist and microbiologist and GZ is biostatistician. Acknowledged is Dr Mukesh Kumar, PhD, Brij Consultants, Maryland, USA for assistance with protocol development and statistical analysis plan.
The trial is funded by Kibow Biotech Inc. and Kibow Pharmaceuticals LLC under the new live biotherapeutic drug development program. The authors are full time employees of Kibow Biotech Inc.
Citation: Anteyi E, Ranganathan P, Vyas U, Zhao Q, Ranganathan N (2021) A Double-Blind Randomized, Placebo-Controlled Phase 2 Clinical Trial to Evaluate Safety and Efficacy of US APR 2020 in Subjects with Chronic Kidney Disease Stage IV. J Clin Trials. 11:468.
Received: 06-Jul-2021 Accepted: 20-Jul-2021 Published: 27-Jul-2021 , DOI: 10.35248/2167-0870.21.11.468
Copyright: © 2021 Anteyi E, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.