
Journal of Clinical Toxicology
Open Access
ISSN: 2161-0495

ISSN: 2161-0495
Case Report - (2021)
Drug-Induced Liver Injury (DILI) may develop depending on the drug itself, its metabolites or the host immune system. Hydroxychloroquine (HQC) is used prevalently in treatment of the rheumatological diseases (especially SLE), and severe liver toxicities associated with this drug have been reported in the literature. We are presenting a 36-year-old patient who was using hydroxychloroquine due to adult Still’s disease (ASD) whose initial values were normal but arrived three weeks later with severely high liver enzyme and bilirubin levels. The diagnosis of the patient was made by eliminating other causes and conducting a liver biopsy. The patient recovered with steroid treatment. This should not be neglected as severe liver failure may develop, even though rarely, in patients using this drug, and this drug is prevalently being used in treatment of coronavirus disease-2019 (COVID-19) especially due to the recent pandemic of coronavirus.
Hydroxychloroquine; Acute liver failure; Adult Still’s disease; Covid-19
Drug-induced liver injury (DILI) may develop through different mechanisms after usage of various prescribed or over-the-counter drugs [1]. This is a complicated process that includes the drug itself, its metabolites and the host immune system [2]. DILI is generally characterized by hepatocellular damage, but it may also induce cholestatic damage or mixed-type damage (containing both hepatocellular damage and cholestatic damage characteristics) [3]. Drug-induced hepatotoxicity may develop due to dose-dependent (predictable) or idiosyncratic (unpredictable) mechanisms. Idiosyncratic reactions may be immune or non-immune. Hydroxychloroquine sulfate, which is an antimalarial drug, is among the drugs that are prevalently prescribed for cutaneous and joint- related symptoms of autoimmune diseases. It is also a drug that is preferred in second-step treatment of adult Still’s disease. Recently, it has started to be used effectively especially for treatment of coronavirus disease -2019 (COVID-2019) [4]. Hydroxychloroquine (HCQ), which is an a-hydroxylated derivative of chloroquine, is usually preferred over chloroquine as its tolerability is higher and toxicity is lower. This drug has a high hepatic metabolism and broad distribution volume especially in the liver, spleen, kidneys, lungs and melanin-containing tissues [5].
Although HCQ may lead to mild gastrointestinal symptoms, as well as high liver enzymes in especially diseases like porphyria cutanea tarda, it is usually well-tolerated. In this article, we discussed a case that was using hydroxychloroquine sulfate for adult Still’s disease and developed severe acute hepatitis in connection to this drug by also reviewing the literature.
36-year-old female patient, presence of complaints of jaundice and itching for 10 days. The patient visited the rheumatology polyclinic with this complaint. Upon detection of high bilirubin and liver enzyme levels in the tests carried out there, she was referred to our clinic. In the patient’s history, she was diagnosed with Adult Still’s Disease (ASD) at the rheumatology polyclinic 1 month before, and treatment of 200 mg/day hydroxychloroquine sulfate and prednisolone 10 mg/day was started. In her physical examination, the sclerae and skin had an icteric appearance, there was mild upper right quadrant pain in her abdominal examination, and other system examinations were normal. In the laboratory tests conducted on the patient, there were high INR (International Normalized Ratio):1.25, AST:693 U/L, ALT:1325 U/L,total bilirubin:10.35 mg/dL, direct bilirubin:9.26 mg/dL, Alkaline phosphatase:135 U/L, GGT(Gamma Glutamyl Transferase):200 IU/L and LDH(Lactate Dehydrogenase): 497 U/L. The patient was hospitalized at the gastroenterology service with the pre- diagnosis of acute hepatitis. All typical and atypical acute hepatitis factors were requested for the patient, and the results were negative. Autoimmune hepatitis tests were requested and came out negative. Abdominal ultrasonography and magnetic resonance cholangiography due to cholestasis were requested, and the results came out normal. In terms of rheumatological involvement, the patient was consulted to rheumatology, but it was found that the patient was in remission in terms of ASD, and there were no clinical and laboratory findings that would indicate other rheumatological diseases. After this, as also the tests of the patient before the Still’s treatment started were normal, the patient was assessed as toxic hepatitis. It was considered that hydroxychloroquine, which was stopped at the hospitalization of the patient at the clinic, could be the drug that led to this situation. Liver biopsy was applied on the patient for pathological diagnosis purposes. In the follow up, the patient had liver enzymes of over 1000, total bilirubin: 22.3 and direct bilirubin: 19.69, the INR value of the patient rose up to 1.95, and referral of the patient was considered by contacting the liver transplantation center. At this point, by considering that the possible toxic effect on the patient was formed immunologically, methyl prednisolone 40 mg/day treatment started, and the laboratory values in the follow ups dramatically recessed. Meanwhile, the patient’s pathology results came out, and around some portal areas, lymphocyte, occasional polymorphous leukocyte, rare eosinophil, focal biliary destruction (cholangitis, ductular proliferation) and occasional restrictive membrane damage could be observed. In the lobular area, hydropic degeneration, edema, occasional rosette-like appearance, lymphocyte, occasional polymorphous leukocyte, 3-4 spotty necrosis in 10x magnification in the most abundant area and bridging confluent necrosis areas around the central vein, in the parenchyma and focal point were observed, and this was reported in compliance with acute toxic hepatitis. The patient who was considered for referral to the liver transplantation center dramatically recovered after steroid treatment and was discharged (Figure 1).
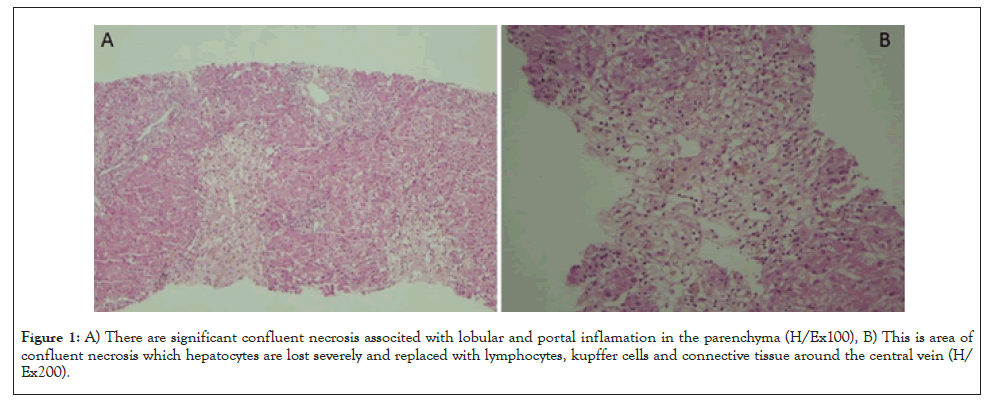
Figure 1: A) There are significant confluent necrosis associted with lobular and portal inflamation in the parenchyma (H/Ex100), B) This is area of confluent necrosis which hepatocytes are lost severely and replaced with lymphocytes, kupffer cells and connective tissue around the central vein (H/Ex200).
High liver enzyme levels are mainly found in the form of a mild- moderate increase in transaminases in 43% to 76% of patients during the course of ASD. However, it was reported in cases that developed acute liver failure [6]. In our patient, it was initially considered that the Still’s disease could have liver involvement, but this was excluded by rheumatology consultation. This was because, as a result of rheumatological assessment, it was reported that the patient was in clinical and laboratory remission in terms of the Still’s disease, and in this case, liver failure was not expected. In Still’s disease, liver dysfunction frequently reflects an underlying disease activity [7]. In our patients, other causes that could induce high bilirubin and liver enzyme levels were examined. Typical and atypical hepatitis factors and autoimmune hepatitis serology were negative. There was no history of alcohol or an underlying disease. In the patient’s history of medication, it was learned that hydroxychloroquine was started 1 month ago with the diagnosis of Still’s disease, and the patient had not used herbal or other medication besides this drug. Additionally, the normal blood values of the patient before using medication supported the possibility of toxic hepatitis. More than 1000 drugs and herbal products lead to DILI development. In the United States of America, the most frequently encountered drug in cases of acute DILI is acetaminophen, which is followed by antibiotics. Worldwide, amoxicillin-clavulanate is one of the most frequently reported causes of DILI [8,9]. Women may be more susceptible to DILI than men, which may in part be due to their generally smaller size [10]. HCQ is used in the entire world in the prophylaxis and treatment of malaria and treatment of rheumatoid arthritis, lupus erythematosus and ASD, and recently, it is being researched in treatment of COVID-19. Even though rarely, this drug is associated with hepatotoxicity, and it largely causes high liver enzyme levels related to idiosyncratic toxic effect. So far, in the literature, a few severe liver injury cases associated with hydroxychloroquine have been reported (Table 1) [11-13]. This case was the third case in the literature that showed acute liver injury findings associated with HCQ usage. All patients were women, and their mean age was 26.4 (16-36). The time between starting medication and emergence of symptoms was 8 hours-1 year. It was confirmed by liver biopsy that three of these patients had drug-induced toxicity. Severe liver failure developed in 3 of these 5 patients including our patient, one received liver transplantation, one died before liver transplantation could be made, and one recovered completely [11-13]. Considering the cases that recovered, starting steroid treatment after stopping HCQ was important in normalizing liver functions and enzymes in the patients. Although glucocorticoids play a role in treatment of excessive sensitivity reactions associated with drugs, they have unproven benefits for most forms of drug-induced hepatotoxicity [14]. We also started steroid treatment in our patient, and she recovered completely in a short time.
| Makin et al. | Galvan et al. | Abdel Galil | This Case | ||
|---|---|---|---|---|---|
| Age | 27 | 16 | 26 | 28 | 36 |
| Sex | Female | Female | Female | Female | Female |
| AST | - | 544 | 399 | 745 | 693 |
| ALT | 2575 | - | 285 | 987 | 1325 |
| Total bilirubin | 9.5 | 24.3 | - | - | 10.35 |
| INR | 3.3 | 3.5 | - | - | 1.25 |
| Post-treatment toxicity findings | After 2 weeks | After 2 weeks | After 8-10 hours | After 1 year | After 3 weeks |
| Symptoms | Nausea, vomiting, jaundice | Jaundice, weakness | Nausea, vomiting, fever | Nausea, vomiting, abdominal pain | Jaundice |
| HCQ dose of usage | 2x200 mg | 2x200 mg | 1x200mg | 1x400 mg | 1x200 mg |
| Disease | SLE | Juvenile still’s disease | Mixed connective tissue disease | SLE | Adult still’s disease |
| Treatment given | none | Liver transplantation | 1x60 mg methyl prednisolone | mycophenolate mofetil+ prednisolone | Prednisolone 40 mg/day |
| Liver biopsy | Performed | Performed | Not performed | Not performed | Performed |
| Outcome | Exitus | Exitus | Recovery | Recovery | Recovery |
Abbreviations: AST: Aspartate Aminotransferase; ALT: Alanine Aminotransferase; INR: International Normalized Ratio; HCQ: Hydroxychloroquine
Table 1: Summarizes the main characteristics of the patients.
Consequently, HCQ may lead to liver failure and even death, even though rarely. For this reason, calling patients whose HCQ treatment started for polyclinic follow up in terms of liver toxicity in the first ten days will allow early determination of possible toxicity. Additionally, steroids may be successfully utilized in treatment of liver toxicity associated with this drug. Especially because it is one of the firstly preferred drugs in recent times for COVID-19 treatment, close follow up of patients in terms of HCQ-related liver toxicity is recommended.
Citation: Gisi K, İspiroglu M, Kilinc E (2021) A Hydroxychloroquine-Related Acute Liver Failure Case and Review of the Literature. J Clin Toxicol. S16:004.
Received: 05-Mar-2021 Accepted: 19-Mar-2021 Published: 26-Mar-2021 , DOI: 10.35248/2161-0495.21.s16.e004
Copyright: © 2021 Gisi K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.