Journal of Nanomedicine & Biotherapeutic Discovery
Open Access
ISSN: 2155-983X
ISSN: 2155-983X
Research Article - (2012) Volume 2, Issue 4
As a natural living system, mammalian cells have the ability to respond to various environmental cues in
distinct ways at both the cellular and molecule levels. Such unique ability of the cells can be utilized for the
rapid detection of chemical and biological analytes. In this report, we have demonstrated the feasibility of a novel cell-based biosensor that utilizes a microcantilever to convert a cellular response to a measurable mechanical response. With this innovative approach, we have been able to detect the distinct responses of α-cyclodextrin and methyl-β-cyclodextrin with mammalian cells based on their differential effects on cells. This study has established a foundation for the future development of a highly sensitive sensing platform for environmental, medical, toxicological, and defense applications.
Keywords: Microcantilever; Nanomechanical; Biosensor; Cellbased assay; Cyclodextrins; Biosensing; Environmental monitoring; Transducer; A431 Cells; Bioanalytical
αCD: α-cyclodextrin; MβCD: methyl-β- cyclodextrin
Biosensors are a type of analytic devices that utilize sensing elements with biological natures, such as antibodies, nucleic acids, enzymes, peptides, and cells, for rapid detection of chemical and biological analytes [1]. In recent years, with live cells as the sensing element, cellbased biosensors have emerged as powerful tools for environmental, medical, toxicological, and defense applications [2,3]. Like other biosensors, cell-based biosensors have characteristics in high selectivity and sensitivity and rapid response towards biologically active analytes. What distinguishes cell-based biosensors from all other biosensors is that they are capable of revealing functional information that is more comprehensive and physiologically relevant. Consequently, cell-based biosensors can provide further insights into the mechanistic basis of actions of analytes in signaling transduction, protein synthesis, cell apoptosis, migration, and metabolism.
Another key part of biosensors is the transducer, which converts the interaction of an analyte with the sensing element into a measurable signal. The transduction efficiencies of the transducers are responsible for the signal stability, reproducibility, sensitivity, and selectivity of biosensors. Many types of transducers have been developed over the years. Electrochemical [4], optical [5], gravimetric/acoustic [6], thermal [7], and mechanical transducers [8] are some of more popular ones.
Microcantilevers [9-11] are highly sensitive nanomechanical devices, evolving from micro-fabricated atomic force microscopy probes that are made of silicon or silicon nitride in a variety of shapes. Typical dimensions are 100-400 μm in length, 1-3 μm in thickness, and 20-50 μm in width. When modified with a biosensing element, the microcantilever can be used as the transducer of a biosensor. For sensing applications, a microcantilever converts the response of the sensing element, which is often in direct contact with the surface of the microcantilever, to a measurable signal in the form of the microcantilever bending or the change in resonance frequency of the microcantilever.
Figure 1 is a schematic drawing that illustrates the detection with a cell-based microcantilever sensor based on the microcantilever bending. The biosensing element (i.e., cells) adhered on one surface of the cantilever responds to an analyte and causes the change of the mechanical properties of the microcantilever beam, which leads to the bending of the microcantilever. The deformation of this cell-adhered microcantilever can be measured by a laser beam reflecting from the tip of the microcantilever. The deformation of the microcantilever ΔZ, in the range of 10-6 to 10-12 m, can be related to the change in surface stress through the Stoney equation [12],
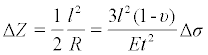 (1)
(1)
Figure 1: Schematic drawing of cell-based microcantilever sensing. (A) The cells exhibit a flat morphology prior to exposure to an analyte. (B) The cells exhibit a more rounded morphology after exposure to an analyte. The deflection of the microcantilever upon the morphological change of the cells is indicated in (B).
where R is the radius of the curvature of the microcantilever; υ is Poisson’s ratio and E is Young’s modulus for the substrate of the microcantilever; l is the length of the microcantilever; t is the thickness of the microcantilever, and Δσ is the differential surface stress.
Although the number of applications of microcantilever biosensors has shown a steady increase over the last 15 years [13], the development of microcantilever biosensors with cell-based sensing elements has been very limited. To our knowledge, the report by Antonik and coworkers back in 1997 is the only published work in this area [14]. In the report, the authors were able to culture live Madin–Darby canine kidney (MDCK) cells on one side of microcantilevers. They observed the deflection of these cell-adhered microcantilevers in the presence of melittin, a powerful stimulator of phospholipase A2, and sodium azide, a cytotoxic agent, respectively. The focus of their report was apparently on the development of a selective culturing method and not on the microcantilever assay, evidenced by the fact that no control of any kind was reported for the bending study. In addition, the level of the deflection presented in an arbitrary unit was not clearly defined. Thus it is unclear if the reported deflection was significant.
In the work reported here, we have developed a simple protocol for preparing a microcantilever biosensor by culturing human epidermoid carcinoma A431 cells on one side of a microcantilever. We have observed distinct real-time responses of the cell-adhered microcantilever to α-cyclodextrin and methyl-β-cyclodextrin. With this work, we have demonstrated the feasibility of cell-adhered microcantilevers in sensing applications and provided direct evidence to address the biological relevance of the observed deflection.
Reagents and material
Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), antibiotics, trypsin–EDTA, HEPES buffer, and HBSS buffer were purchased from Invitrogen (Carlsbad, CA). The human epidermoid carcinoma A431 cell line was obtained from American Type Tissue Collection (Manassas, VA). α-Cyclodextrin (αCD) and methyl-β-cyclodextrin (MβCD) were obtained from Sigma-Aldrich (St. Louis, MO). Silicon microcantilevers (CSC38/Cr-Au, length / width / thickness = 350 μm /35 μm / 1.0 μm, force constant 0.03 N/m) were purchased from MikroMasch (Wilsonville, OR). Both sides of the microcantilever have a thin film of chromium (3 nm) followed by a 20 nm layer of gold deposited by e-beam evaporation.
Cell culture
A431 cells were cultured in T75 Corning culture flasks and maintained under a humidified atmosphere at 37°C and 5% CO2 in DMEM containing 10% FBS, 100 IU/mL penicillin, and 100 μg/mL streptomycin. The cells were usually harvested at 95% confluency.
The microcantilevers were cleaned with washes with water and ethanol, followed by exposure to UV-ozone for 20 minutes. They were then placed into a well in a 12-well tissue culture plate along with A431 cells (1.6 x 106 cells/mL) that were harvested from the T75 culture flask. The cells were allowed to adhere and grow on the front (non-reflective) side of the microcantilevers under a humidified atmosphere at 37°C and 5% CO2. Once they reached 80-90% coverage, the adherent cells on the surface of the microcantilevers were washed with phosphate buffered saline and starved in serum free medium in a different well of the same 12-well plate for 18 hrs before used.
Microcantilever assays
On the day of the assay, the cells adhered on the microcantilever were carefully rinsed with the assay buffer (20 mM HEPES in HBSS buffer, pH 7.2) and the microcantilever was then placed in a flowthrough glass cell with a volume of 0.3 mL. The microcantilever assay was then performed as described previously [15]. In brief, a constant flow of the assay buffer through the flow cell was maintained at 1 mL/h with a syringe pump to minimize the baseline fluctuation. Once a stable baseline was obtained, an analyte dissolved in the assay buffer was injected through the injection port equipped with a sample loop of 0.5 mL into the flow cell. The deflection of the microcantilever was measured based on the position of a laser beam reflected from the microcantilever onto a four-quadrant photodiode (Figure 1). Each assay was repeated multiple times to ensure its reliability and reproducibility.
In this study, we investigated the feasibility of cell-adhered microcantilevers in biosensing, more specifically, the ability of a microcantilever to convert the response of the adhered cells into a measurable mechanical response (i.e., bending). Since the microcantilever bending is caused by the unbalanced surface stress between the front and back sides of the microcantilever, it is critical to culture the cells selectively on one side of the microcantilever. First, we prepared the cell-adhered microcantilever by selectively culturing human epidermoid carcinoma A431 cells onto the front side (non-reflective side) of the microcantilever (Figures 1 and 3A). Due to the strong adherent property of the cells, A431 cells formed a uniform monolayer which is ideal for such a cell-based assay. This was readily achieved by facing the backside of the microcantilever (reflective side) down in a tissue-culture well. The cells fell on the face-up front-side surface only during the seeding process. Another important contributing factor to sensitivity is the surface coverage of the microcantilever with the cells. Satisfactory surface coverage (Figure 3A) was obtained when a high concentration (1.6 x 106 cells/mL) of the cells was used.
The sensing ability of cell-adhered microcantilevers was investigated by exposing the microcantilevers to methyl-β-cyclodextrin (MβCD) and α-cyclodextrin (αCD) (Figures 2A and 2B). Both compounds belong to cyclodextrins, a family of cyclic oligosaccharides composed of α-1, 4-linked glucopyranose subunits. The difference between the two is that αCD has six glucopyranose subunits whereas MβCD has seven, which results in different sizes of hydrophobic cavities [16]. Figure 4 showed the deflections of the microcantilevers exposed to MβCD and αCD, respectively. In these experiments, first, a cell-adhered microcantilever was placed in a flow cell and allowed to equilibrate at room temperature with a constant flow of the assay buffer at 1 mL/h. Once a stable baseline was obtained, MβCD or αCD was injected into the flow cell. It took approximately 15 min for the solution to flow into the cell at this flow rate. For MβCD, the bending response remained at a deflection rate of 0.27 nm/min for the first 20 min, and approximately 0.54 nm/ min in the next 50 min. This rate increase could be an indication of the occurrence of a second cellular response at a higher concentration of MβCD. This phenomenon was also observed for αCD. However, the overall deflection of the microcantilever when exposed to αCD, was much smaller compared to that of MβCD. A more quantitative comparison shows that the rate of deflection for αCD is about 5% of that for MβCD. The larger microcantilever deflection and faster rate of deflection suggests larger surface stress change on the microcantilever surface on exposure to MβCD. For the 35 nm maximum deflection on exposure to MβCD, the surface stress change was 0.02 N/m according to equation 1.
We attribute the observed large bending of the microcantilever in Figure 4 to the MβCD-induced cellular response. MβCD is known to extract cholesterol from cell surface by forming inclusion complexes. One of the important functions of cholesterol is to maintain the integrity and fluidity of cell membrane, and secure important proteins in membrane. By disrupting the structure of cholesterol-rich microdomains, MβCD was able to change the cell morphology to a more rounded shape, which was evident in Figure 3B. Consequently, the interaction between individual cells and the surface of the microcantilever was substantially altered, which conceivably led to the significant change of the overall surface stress of the microcantilever and the subsequent large bending of the microcantilever. Thus, the cell-adhered microcantilever was able to convert the MβCD-induced cellular response to a measurable mechanical response.
Although both αCD and MβCD are cyclic oligosaccharides composed of α-1, 4-linked glucopyranose subunits, αCD does not extract cholesterol from cells as well as MβCD [16,17], because αCD has a smaller hydrophobic cavity compared to that of MβCD. In fact, αCD is often used as a negative control for MβCD in the study involving cholesterol extraction of cells [18]. As a result, αCD was unable to cause a substantial change in cell morphology, which probably attributed to the slower deflection rate compared to that of MβCD.
Overall, this study has for the first time demonstrated the feasibility of cell-based microcantilever sensing, which capitalizes the unique capability of microcantilever to convert cellular response to a measurable mechanical response. Compared to other sensing technologies, cell-based microcantilever sensing is a label free, noninvasive technology that provides a real-time monitoring capability based on the induced cellular response. In addition, it has a good specificity with a low production cost, low-power consumption, and a small size [13]. Cell-based microcantilever sensing has the potential to become a cost-effective and highly sensitive sensing platform for environmental, medical, toxicological, and defense applications.
This work was supported by National Institutes of Health (NIH) Grant Number 1R01NS057366.