Clinical & Experimental Cardiology
Open Access
ISSN: 2155-9880
ISSN: 2155-9880
Research Article - (2022)Volume 13, Issue 4
Background: Hypertrophic cardiomyopathy (HCM) is a common genetically based cardiac disease with an estimated prevalence of 1 in 200-500 cases that poses a significant threat to young adults and athletes. The genetic basis of HCM involves sequence variations in several genes that encode proteins of the thick and thin cardiac myofilaments which are responsible for the contraction of the cardiac sarcomere. Pathogenic mutations that cause hypertrophic cardiomyopathy are transmitted in an autosomal dominant pattern. HCM is generally stratified into obstructive (about 70%) and non-obstructive hypertrophic cardiomyopathy. Clinical manifestation of HCM can range from asymptomatic to drug-refractory advanced heart failure. Mavacamten is a first-in-class myosin inhibiting drug that has progressed through in vitro studies. It has shown promising results in patients with symptomatic hypertrophic obstructive cardiomyopathy.
Objectives: The purpose of this study was to conduct a systematic evaluation and outcome assessment of published and on-going studies of mavacamten therapy to treat HCM.
Methods: The databases PubMed, EMBASE, Clinicaltrials.gov, and Medline were searched with keywords for the existing literature on mavacamten for treating HCM. Cross-referencing was used to determine the eligibility of retrieved articles and to identify biases.
Results: A total of 1066 studies were found in an initial keyword search. These articles were then subjected to an eligibility criterion to ensure relevance to the review objectives. Stratification of possible publications identified 9 studies for inclusion in the review, including randomized clinical trials, Clinical Trials, and ongoing Trials. A metaanalysis of probable mavacamten outcomes was then undertaken using the Cochrane Meta-analytic Tool, with the results visualized as forest plots and a narrative table.
Conclusion: Treatment of symptomatic obstructive cardiomyopathy with mavacamten significantly impacted primary outcomes, such as improved left ventricular obstruction tract gradient and increased peak oxygen consumption, as well as secondary outcomes, such as improved exercise resilience, reduced NYHA classes, increased life-years, and improved overall quality of life.
Mavacamten; Myosin inhibitors; Hypertrophic obstructive cardiomyopathy
Hypertrophic Cardiomyopathy (HCM) is a somewhat common genetic cardiac disorder characterized by alterations in cardiac muscle structure resulting in compromised cardiac function. With an estimated prevalence of 1 case per 500 to 1000 individuals, the disease poses a significant threat particularly to young adults and athletes [1]. HCM is mainly associated with myocardial hypertrophy with a dimension exceeding 15 millimeters. This hypertrophy exists in the absence of pressure loads or myocyte disarray [2-4]. HCM is generally stratified into two categories; obstructive and non-obstructive hypertrophic cardiomyopathy [1].
Hypertrophic Obstructive Cardiomyopathy (HOCM) is characterized by excessive thickening of the left ventricular myocardium and dynamic left ventricular outflow tract obstruction, which leads to obstruction or blockage of the outflow tract of the left ventricle. This blockage stems from various factors, such as the hypertrophy of the interventricular septum and the systolic anterior movements of the mitral valve [5]. Inhibition of the left ventricular outflow tract results in symptoms that put limitations on patients’ daily physical activities. HOCM is the most common form of HCM and is present in over 70% of all recognized HCM [1].
The genetic basis of HCM involves sequence variations in several genes that encode proteins of the thick and thin cardiac myofilaments (B-myosin heavy chain, troponin, actin, and titin) or the adjacent Z-Disc, which are responsible for the contraction of the cardiac sarcomere Pathogenic mutations that cause hypertrophic cardiomyopathy are transmitted in an autosomal dominant pattern, with 50% chance of inheritance for every off spring [2,4].The majority of patients with nonobstructive HCM remain asymptomatic or mildly symptomatic and experience little or no functional disability. Additionally, patients with nonobstructive HCM are at low risk for progressive heart failure (NYHA functional classes III/IV) at 1.6% per year, compared with 3.2% per year in patients with provocable obstruction and 7.4% per year in patients with obstruction at rest [6].
Ho, et al. suggest that the clinical manifestation of HCM can occur at an early age and that patients who are diagnosed early may be at increased risk of more severe outcomes, especially those with specific pathogenic sequence variants in sarcomere genes [7]. The clinical presentation of HCM can vary from asymptomatic to heart failure and possible death. Physical examination of individuals with HCM can reveal high-pitched, crescendodecrescendo mid-systolic murmur at the left lower sternal border that becomes louder with the Valsalva maneuver. Electrocardiogram can show large, dagger-like septal Q waves in the inferior and lateral leads due to an abnormally hypertrophied interventricular septum. Sudden cardiac death due to ventricular arrhythmias associated with intense physical activity occurs in about 1% of HCM patients annually [8]. A concerted effort is being made to identify high-risk patients with an effect already in place, as witnessed in the defibrillator implantation in high-risk patients [9,10].
This group include patients with HOCM and any of the following:
• Syncope
• Interventricular septal thickness of 30 mm or greater
• Documented ventricular tachycardia and/or cardiac arrest
• Family history of sudden cardiac death
• Left ventricular systolic dysfunction in the setting of wall thinning, also known as “burnt out” left ventricle
Morbidities associated with HOCM include heart failure, decreased quality of life, and atrial fibrillation, present in 1 out of 5 symptomatic patients. Moreover, HOCM is associated with symptoms such as dyspnea, chest pain, angina, fatigue, palpitations, syncope, and reduced or nontolerance to exhausting physical activities [11,12]. Existing medical interventions for symptomatic HOCM patients are beta-blockers and nondihydropyridine calcium channel blockers [7,12]. These treatments carry the risk of side effects or an impaired efficacy level.
The American College of Cardiology Foundation and the European Society of Cardiology recommend invasive treatment approaches such as surgical septal myectomy or alcohol septal ablation in patients exhibiting failure to response to the optimal medical treatment with a Left Ventricular Outflow Tract (LVOT) gradient measuring above 50 mm Hg [11,12]. However, these invasive procedures expose the patient to risks inherent to surgical approaches, such as risk of infection, high cost, and availability surgeons who are competent to perform such procedures, which greatly limits patients’ access to optimal therapy [2,8].
The last few years have seen a combined effort by healthcare research institutes to engineer drugs for treating HCM as an alternative to the traditional invasive surgical procedures. These include exploring myocardial fibrosis modulating drugs such as spironolactone, which unfortunately failed in a randomized controlled trial conducted by Maron, et al. [12]. Mavacamten, formerly called MYK-461, became the first myosin inhibitor to pass the in vitro study phase for treating HCM. Mavacamten is a molecule that was discovered through analysis and stratification of compounds that reduce ATPase activity in cardiac myosin [13]. An analysis into the working mechanism of mavacamten by Green et al. showed that the molecule led to a decrease in the phosphate releasing rate and, as a result, increased the duration of myosin relaxation [13] (Figure 1).
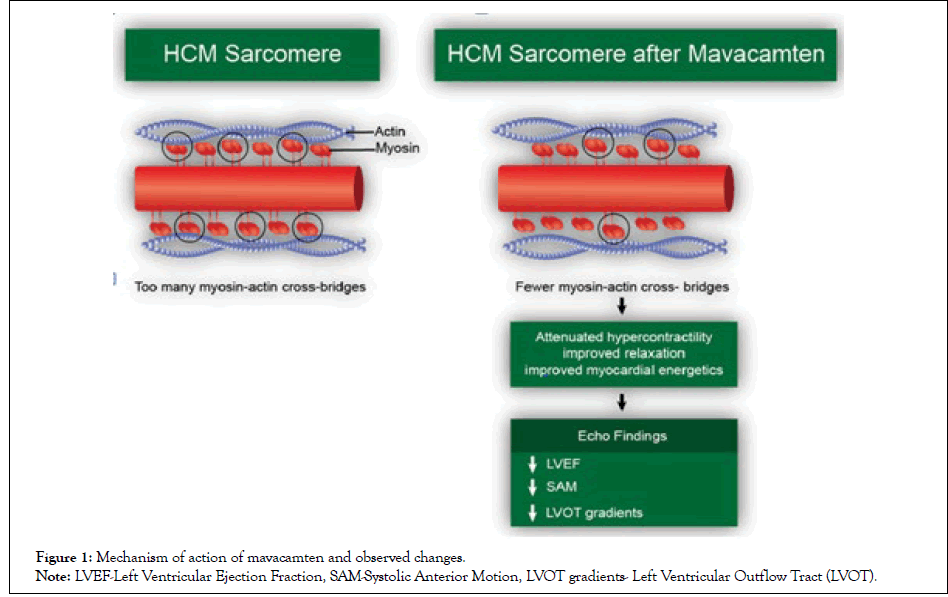
Figure 1: Mechanism of action of mavacamten and observed changes.
Note: LVEF-Left Ventricular Ejection Fraction, SAM-Systolic Anterior Motion, LVOT gradients- Left Ventricular Outflow Tract (LVOT).
Comprehensive studies of mavacamten in humans have shown that it alters myocardial relaxation and hyperdynamic contractions in HCM patients who have the MYH7 and MYBP3 genotypic variants. This Meta- Analysis aims to assess the current state of research regarding the benefits of mavacamten in patients with HCM, particularly those with HOCM [7,14]. This medication administered orally as it is a capsule with multiple strength including: 2.5 mg, 5 mg, 10 mg, and 15 mg. Contraindications are Moderate to strong CYP2C19 inhibitors or strong CYP3A4 inhibitors (4, 5, 2). Moderate to strong CYP2C19 inducers or moderate to strong CYP3A4 inducers. it can cause heart failure due to systolic dysfunction. Thus, echocardiogram assessments of left ventricular ejection fraction (LVEF) required before and during use. initiation in patients with LVEF <55 % is not recommended. Need to interrupt if EF <50% or worsening clinical status [15]. This Meta- Analysis aims to assess the current state of research regarding the benefits of mavacamten in patients with HCM, particularly those with HOCM.
Search strategy
Between January 2020 and November 2021, a comprehensive review and literature analysis were conducted. The following medical databases were searched: Cochrane Database of Systematic Reviews, MEDLINE, EMBASE, PubMed, Google Scholar, and SCOPUS. Electronic searches were conducted in Clinical Trials, the ISRCT, and the International Prospective Register of Systematic Reviews for any ongoing studies on the subject. Manual searches were also conducted on references cited in the examined publications.
The above databases were queried with the following keywords: Mavacamten, treatment, hypertrophic obstructive cardiomyopathy. To retrieve literature published between 2000 and 2022, we used the English language, chronological criteria in the search method.
Inclusion and exclusion criteria
• Studies included in this systematic review had to adhere to the following inclusion criteria: reported risk estimates; reported novel research results; dated from 2020 to the present; were randomized controlled trials on mavacamten treatment of HOCM.
• Studies dating from the year 2020 onwards
• Random Control trials, RCTs, on the administration of Mavacamten to treat HOCM
Study selection and data synthesis
A structured and systematic review of the various data sources was conducted. To determine which studies should be included and which should be excluded from consideration, the inclusion and exclusion criteria were applied in conjunction with the various databases listed above. Divergence between the data and the information provided by the main author were also examined. In cases where the information provided by the primary author could not be retrieved, the chief researcher independently validated the discrepancies. All full-length articles approved for consideration were chosen and analyzed by each researcher impartially. The group agreed to provide data with the greatest degree of transparency possible, and any conflicts that developed were gauged and determined as a consequence of the agreement (Figure 2).
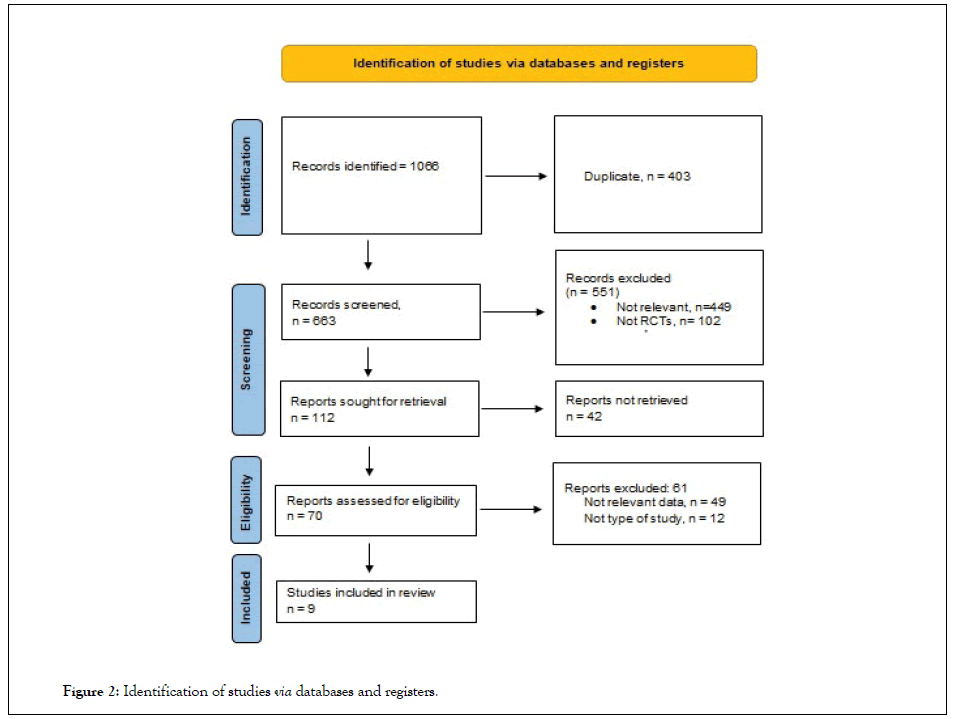
Figure 2: Identification of studies via databases and registers.
Data analysis
A systematic narrative synthesis was utilized. The review also adopted the use of synopses and tabulation of the studies. Specifically, we looked for effect estimates that (1) captured data for symptomatic adult (≥ 18 years) patients with HOCM; (2) measured LVOT gradient and included the NYHA classes, (3) made measurements before, during, and after exercise and assessed quality of life influences of mavacamten treatment; and (4) measured the effect of treatment on potential life years of symptomatic HOCM patients (Figures 3 and 4).
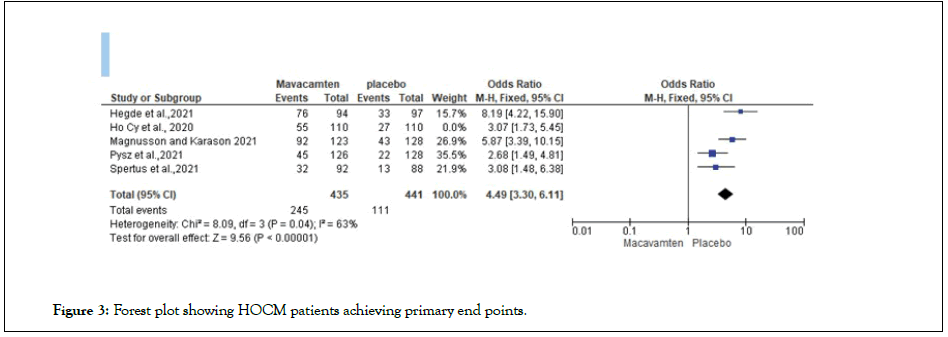
Figure 3: Forest plot showing HOCM patients achieving primary end points.
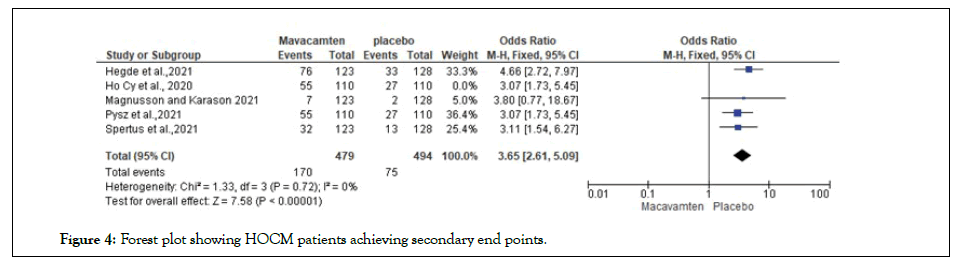
Figure 4: Forest plot showing HOCM patients achieving secondary end points.
Risk of bias
Obscured randomization, specified inclusion and exclusion parameters, the blinded study considered, individual screening, blinded data processing, and intentions to treat analysis were all employed to reduce bias. The healthcare population providing the treatments could not be blinded. The overall risk of bias in the studies was assessed using the Cochrane Handbook Tool for Risk of Bias. The risk of bias for the studies was determined as having a high, low, or unclear risk of bias.
Figure 5 below represents data derived from sequence generation,
allocation concealment, blinding of participants, personnel and outcome
assessors, incomplete data, selective outcome reporting, and other risks are
as addressed in the Cochrane tool. 
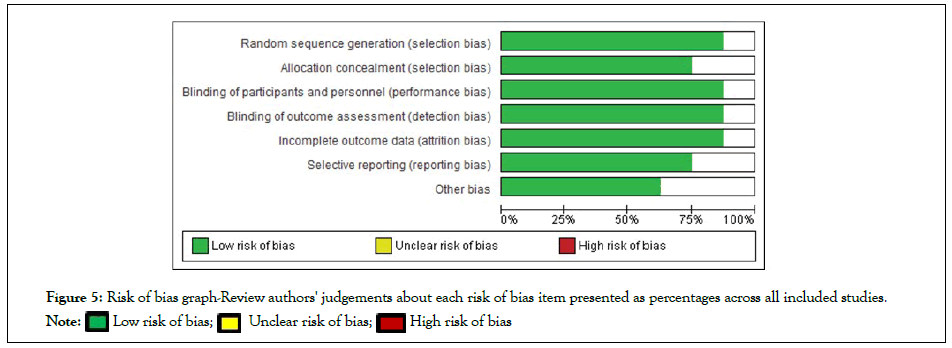
Figure 5: Risk of bias graph-Review authors' judgements about each risk of bias item presented as percentages across all included studies
Two reviewers independently assessed the quality of included studies. An assessment of the RCTs included in the study revealed an insignificant amount of bias as most of the studies have reported both primary and secondary outcomes associated with mavacamten treatment of obstructive hypertrophic cardiomyopathy.
The randomized nature of conduction of the RCTs in the study of mavacamten as a treatment option for obstructive hypertrophic cardiomyopathy meant there was little interference in the selection of the treatment population. an unclear risk of bias was represented in effect to randomization of a small intervention sample. Of the 9 included studies, 3 were ongoing trials; therefore the other 6 studies underwent quality risk assessment [16-21]. All of them were at low risk of bias under the random sequence generation, blinding of participants and personnel, blinding of outcome assessment, and incomplete outcome data. For allocation concealment, 5 studies were of low risk and one of unclear risk. For selective reporting bias, 5 studies were of low risk, one was of unclear risk. For other bias, 5 studies were of low risk, 2 were of unclear risk (Figure 6).
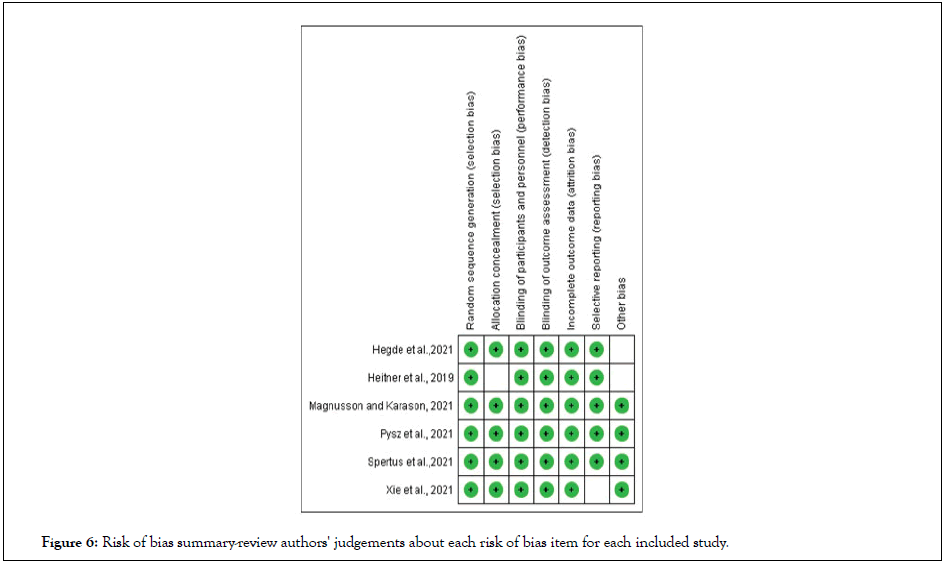
Figure 6: Risk of bias summary-review authors' judgements about each risk of bias item for each included study.
1066 studies were found, 403 were duplicated. We were left with 663 studies for screening, 551 were excluded as 449 studies were non relevant, and 102 studies were not RCTs. The rest of 112 studies were further searched and 42 studies were not retrieved. Screening for eligibility was done for the last 70 studies for which 61 studies were excluded either because of non-relevant data (49 studies) or not the desired type of study (12 studies). 9 studies were included in the study [22,23].
In this study we assessed for primary and secondary out-comes after using mavacamten. The primary HCM's end point at week 30 of treatment with mavacamten versus placebo is a composite functional end point defined as achieving either:
• An improvement of at least 1.5 mL/kg/min in peak oxygen consumption (pVO2) as determined by CPET and a reduction of 1 NYHA functional class
• An improvement of at least 3.0 mL/kg/min in pVO2 with no worsening in NYHA functional class; or
Complete resolution of mitral valve systolic anterior motion.
An assessed odds ratio of 4.49[3.30, 6.11] showed a statistically significant improvement in the mavacamten treatment group compared to placebo. A 95% Confidence Interval (CI) was utilized with the studies showing a low heterogeneity (P=0.04) [P<1]
The secondary endpoints include comparisons of mavacamten against placebo in the following parameters from baseline to week 30: Postexercise LVOT gradient, pVO2, NYHA class, and two PROs: pVO2 and pVO2. The Kansas City Cardiomyopathy Questionnaire was used to assess healthrelated quality of life, and the Hypertrophic Cardiomyopathy Symptom Questionnaire was used to assess HCM core symptoms (shortness of breath sub score).
Odds Ratio (OR) calculated on improved secondary end points on the mavacamten intervention group revealed a significant improvement in achievement of secondary end point in the mavacamten group compared to placebo, 3.65 [2.61, 5.09], heterogeneity score on the included RCTs revealed a insignificant score (P=0.72) [P<1].
Scientific invention and ambition in the past few years have led to a greater understanding of HCM pathogenesis. This understanding has been contributed to the shift in treatment of HCM toward pharmacological management and away from more invasive strategies. After thorough testing, myosin inhibitors have a significant role in future HCM management campaigns.
Mavacamten is a myosin-ATPase enzyme-specific inhibitor. This mechanism of action contributes to a reduction in contractility and optimization of energy utilization in the myocytes [24]. Favorable outcomes seen in patients treated with mavacamten in ongoing and concluded studies show that the drug may be effective for treating HOCM.
An analysis of the literature on how mavacamten may become a possible long-term solution to treating HCM has provided useful information. However, several questions remain, and whether incorporating mavacamten into a daily drug regimen will be effective for treating HCM remains a contentious issue among scientists. The ongoing VALOR-HCM (NCT04349072) trial aims to answer the questions regarding the feasibility and effectiveness of mavacamten as a targeted HCM therapy [23].
Mavacamten remains a new and exploratory medication approach for HCM management. Thus, the legitimacy of the drug for long-term use remains a critical issue to be elucidated. The long-term assessment of mavacamten efficacy for treating HCM is being investigated by the ongoing MAVA-LTE (NCT03723655) and PIONEER-HCM (NCT03496168) control trials.
Partial annual results derived from the PIONEER-HCM (NCT03496168) and the EXPLORER-HCM sub-studies clinical trial indicate that mavacamten may lead to a gradual improvement in HCM symptoms, improved LVOT scores, decreased interventricular septum thickness, and significantly decreased left atrial volume. However, the EXPLORER-HCM sub-study did not show any beneficial characteristics from mavacamten therapy in regard to maximization of cardiac muscle thickness, indexed left atrial volume, and indexed left ventricular mass when viewed under a magnetic camera. The sub-study also failed to show a substantial difference between the intervention and placebo groups, which was not an unexpected outcome because of the low rate of disease progression in patients and a limited follow-up period [21].
The majority of the included studies in the systematic review measured primary outcomes according to the phenotypical manifestations of HOCM, including LVOT gradient and myocardial hypercontractility. The secondary outcomes measured included quality of life, exercise tolerance, improvement in the NYHA class index, and the health utility of mavacamten administration to symptomatic HOCM patients. Results from the studies above highlighted the overall improvement in quality of life and health status in the treatment groups compared to the placebo.
Mavacamten was well tolerated in all included studies, with few or no adverse effects observed. Several of the included studies addressed limitations in their studies. These limitations included the lack of a large survey and experimental population and the inability to evaluate of the long-term efficacy of mavacamten administration to symptomatic HOCM patients.
Overall, the significant advantages associated with mavacamten therapy for HCM patients suggests that myosin-inhibitors may be used as alternative treatment interventions for symptomatic HOCM patients in the short-term, and perhaps in the long term, as seen in the improved quality of life and an increase in the life years of symptomatic HOCM patients under mavacamten treatment.
Limitations
Few limitations were found which will need more studies to address them in the future.
• The studies reviewed have very heterogeneous design and outcomes
• The studies you reviewed compare mavacamten vs. placebo, but are the patients on bestmedical Rx at the time of enrollment? The question is whether the drug is better than beta blocker and calcium channel blockers. Still un-clear
• The number of the studies that were included was small and limited, will need more studies in the future.
Despite these limitations we think it is a good study to be published as the data is so promising, and will help bring more attention to the subject for further studies in the near future.
Studies have shown that using the first in class myosin inhibitor mavacamten to treat symptomatic patients who have hypertrophic obstructive cardiomyopathy results in significantly increased exercise endurance, improved NYHA class, improved LVOT gradient, and increased life years through overall reduced mortality rates. Importantly, mavacamten may lead to higher quality of life as measured in terms of health utility for patients with this serious cardiac condition. Mavacamten was well tolerated in all included studies, with few or no adverse effects observed. However, these studies had some limitation which included the lack of a large survey and experimental population, and the inability to evaluate of the longterm efficacy of mavacamten administration to patients with symptomatic hypertrophic obstructive cardiomyopathy.
[Crossref]
Citation: Affas Z, Touza G, Touza R, Affas R, Azzo S, Shakir A (2022) A Meta-Analysis and Systematic Review of Mavacamten a Novel Disease-Specific Treatment for Hypertrophic Obstructive Cardiomyopathy. J Clin Exp Cardiolog. 13:722.
Received: 25-Apr-2022, Manuscript No. JCEC-22-17134 ; Editor assigned: 28-Apr-2022, Pre QC No. JCEC-22-17134 (PQ); Reviewed: 16-May-2022, QC No. JCEC-22-17134 ; Revised: 23-May-2022, Manuscript No. JCEC-22-17134 (R); Published: 30-May-2022 , DOI: 10.35248/2155-9880.22.13.722
Copyright: © 2022 Affas Z, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.