
Journal of Clinical Toxicology
Open Access
ISSN: 2161-0495

ISSN: 2161-0495
Review - (2024)Volume 14, Issue 4
Polybrominated Diphenyl Ethers (PBDEs) is a widely used brominated flame retardant extensively incorporated into various consumer and industrial products. Due to its high stability and resistance to degradation, PBDE-209 persists in the environment for prolonged periods and bioaccumulates in the food chain, ultimately reaching the human body. An increasing body of research has demonstrated that PBDEs exerts significant neurotoxic effects, particularly impacting neurodevelopment and neural function. The neurotoxicity of PBDEs is primarily mediated through mechanisms mainly including oxidative stress, mitochondrial dysfunction, disruption of calcium homeostasis, alterations of thyroid hormone homeostasis, interference with neural differentiation, migration and neurotransmitter systems. Compounds targeting these critical mechanisms might have the capability to reduce the neurotoxicity of PBDEs. This review aims to elucidate the neurotoxic mechanisms of PBDEs and explore effective pharmacological countermeasures. PBDEs exposure is inextricably linked to neurodevelopmental toxicity, involving diverse mechanisms that intertwine and interact. While certain compounds and associated targets have been identified to mitigate this neurotoxicity, extensive human clinical studies are still required to further explore their safety and efficacy.
PBDEs; Developmental eurotoxicity; Mechanism; Pharmacological countermeasures
Polybrominated Diphenyl Ethers (PBDEs), extensively utilized in electronics, textiles, and furnishings for its brominated flameretardant properties, exhibits significant chemical stability and resistance to degradation. Consequently, PBDEs persists in the environment over prolonged periods, bioaccumulating through food chains and ultimately accumulating in human tissues. Increasing studies highlight the significant neurotoxic effects of PBDEs, particularly on neurodevelopment and neural function [1,2]. Consequently, the investigation of effective pharmacological agents to prevent or mitigate the neurotoxic effects induced by PBDEs exposure is of critical significance. PBDE's environmental persistence and bioaccumulation have rendered it a global public health concern. Its biomagnification through the food chain results in significant human body burdens, particularly evidenced by high concentrations of PBDEs in blood, adipose tissue, and breast milk [3]. This phenomenon has raised extensive concerns regarding its long-term health impacts. In this review, we extend knowledge from previous studies of neurotoxicity and underlying mechanisms of adverse effects on the nervous system by exposure to PBDEs and explore potential therapeutic agents.
Neurotoxic effects of PBDEs on humans
Increasing studies have substantiated that PBDEs exerts potential neurotoxic effects on the nervous system, detrimentally influencing both neurodevelopment and neural function. Study by Herbstman et al., found PBDEs in 94% of cord blood samples from 297 Baltimore newborns, with BDE-47 as the dominant congener [4]. This finding raised concerns regarding PBDEs exposure and its potential toxic effects. The inaugural epidemiological investigation conducted by Roze et al., elucidated that prenatal PBDEs exposure correlated with adverse developmental outcomes, including reduced fine manipulative abilities and increased attentional deficits [5]. Research by Herbstman et al., established that higher concentrations of BDEs 47, 99, and 100 in cord blood were linked to lower scores on mental and physical development evaluations at 12-48, and 72 months, and with notable correlations in psychomotor and mental development indices, as well as IQ scores [6]. Gason, et al., measured PBDEs concentrations in colostrum from 290 Spanish women and assessed their infants' mental and psychomotor development at 12-18 months [7]. Higher PBDEs levels, especially BDE-209, were marginally associated with lower mental development scores [7]. The 2013 study by Eskenazi, et al., within the CHAMACOS project provided compelling evidence supporting that prenatal and childhood exposures to PBDEs correlate with reduced attention, motor skills, and cognitive abilities in children, highlighting the adverse effects of PBDEs on neurological development in youth [8]. A 2014 USA study linked prenatal PBDEs exposure to reduced IQ and higher ADHD in children. Specifically, a tenfold increase in BDE-47 levels resulted in a 4.5-point IQ decrease and a 3.3-point rise in ADHD scores by age 5 [9]. Prenatal PBDEs concentration was inversely associated with reading skills and FSIQ and positively associated with externalizing behavior problems at age 8 years [10]. A 2018 study found that prenatal and childhood PBDEs exposure significantly affects memory function in early adolescence, with a more pronounced impact on females [11]. The research in China linked low-level prenatal exposure to PBDEs with long-term behavioral issues in children, including somatic complaints and withdrawal. Girls experienced more pronounced sleep and internalizing problems, while boys showed greater attention issues [12]. Liang, et al., studied part of the HOME project in Cincinnati, measured serum PBDEs levels in 230 children at multiple ages and assessed their reading skills at ages 5 and 8 [13]. They found that higher PBDEs concentrations, including BDE-153, BDE-47, BDE-99, BDE-100, and total of these four (Sum4PBDEs), were linked to lower reading scores, although these associations lost statistical significance after adjusting for other factors [13]. The research conducted by Margoliset, et al., Vuong, et al., yielded congruent findings: Prenatal PBDEs exposure could be a risk factor for reading difficulties in children prenatal exposure to PBDEs is linked to changes in neural activity in brain regions important for inhibitory control, suggesting that these exposures might contribute to ADHD symptoms by altering essential neural functions for cognitive and behavioral regulation [14-17]. Gaylord et al., analysis of data from 2001 to 2016 estimated that PBDEs exposure led to a loss of about 162 million IQ points and contributed to 738,000 cases of intellectual disability, underscoring the significant impact of in utero PBDEs exposure on intellectual outcomes [18]. These studies collectively indicate that prenatal and neonatal exposure to PBDEs is linked to various neurodevelopmental deficits. The relationship between PBDEs exposure and cognitive function is complex and varies across different racial and socioeconomic groups. These findings highlight the need for ongoing research into neurotoxic mechanisms and interventions to mitigate PBDEs exposure risks during critical developmental periods.
Neurotoxic effects of PBDEs on animals
Numerous rodent studies have shown that exposure to PBDEs leads to behavioral changes and brain alterations. Eriksson et al., found that a single dose of BDE-47 and BDE-99 given to mice affected their spontaneous behavior and memory performance in a dose-dependent manner, with observed declines in habituation ability as the mice aged [19]. Markowski, et al., found that male mice exposed to decaBDE from birth to day 21 showed reduced grip strength, altered motor activities, increased errors in learning tasks, and reduced responses to a drug challenge [20]. Exposed female mice primarily showed changes in activity levels. Co-exposure to Polystyrene Nanoplastics (PS-NPs) and BDE-47 disrupts the expression of key genes such as GAP43, TUBA1A, SHHα, MBP, CHRNα7, and AChE in juvenile zebrafish [21]. It is noteworthy that BDE-209 exhibited greater neurotoxic effects compared to other flame retardants such as Hexabromocyclododecane (HBCD) and Tetrabromobisphenol-A (TBBPA) [22]. Co-exposure to PS-NPs and BDE-47 adversely affects neuronal development and the cholinergic system, leading to heightened neurotoxic effects in juvenile zebrafish [23]. Perinatal exposure to PBDE-47 led to increased hyperactivity and possible elevated anxiety in adult female rats, indicated by more movement and less time spent in the central zone of an open field test [24]. The aforementioned studies emphatically indicate that PBDEs exposure is linked to significant neurobehavioral detriments.
Mechanism of neurotoxicity induced by PBDEs
Oxidative stress: Oxidative stress is a pivotal mechanism in PBDE-induced neurotoxicity. Early in vitro studies have demonstrated that Tetra-and PentaBDE, as well as PBDE-209, induce toxicity in primary neurons and neuronal cell lines primarily through oxidative stress, autophagy disruption, and apoptosis [25,26]. Li et al., the interaction between autophagy and apoptosis in PBDE-47- induced neurotoxicity, revealing that disrupted autophagy exacerbates apoptosis, leading to significant neuronal loss and cognitive deficits in rats and wortmannin can reverse the adverse effects of PBDE-47 [27]. PBDE-47 has been shown to cause iron overload, lipid peroxidation, and mitochondrial damage in neuron-like PC12 cells, which are indicative of oxidative stress and ferroptosis which is a novel form of cell death dependent on iron and Reactive Oxygen Species (ROS) accumulation [28]. Additionally, PBDEs quinone metabolites' nucleophilic and redox properties contribute to their neurotoxicity [29]. Research demonstrates that BDE-47 treatment in mice activates caspase-3-dependent PKCδ, which increases ROS levels, causing hippocampal inflammation and cognitive deficits. Inhibition of this pathway using Z-DEVDFMK, N-Acetyl-L-Cysteine (NAC), or DJ-1 overexpression via adeno-associated viral vectors reduces ROS and improves cognitive functions, these substances hold potential as potential therapeutic drugs [30]. These study highlighting the critical role of oxidative stress in PBDE-induced neurotoxicity (Figure 1).
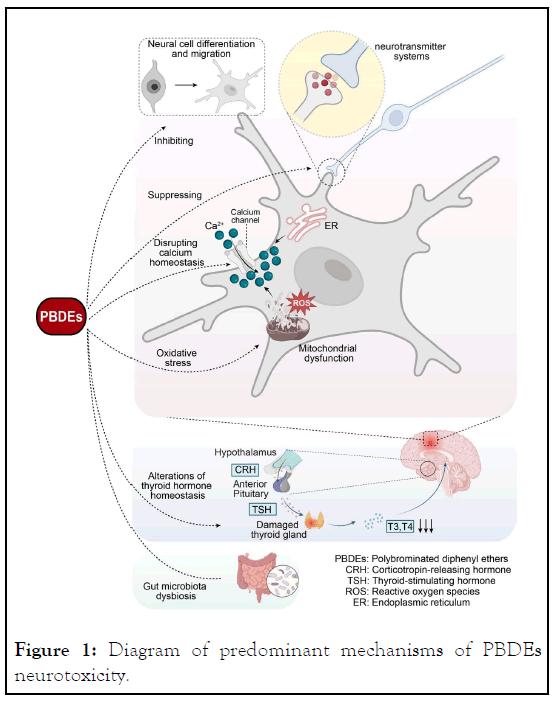
Figure 1: Diagram of predominant mechanisms of PBDEs neurotoxicity.
PBDEs induce neurotoxicity via interference with neural differentiation and migration, neurotransmitter systems, oxidative stress, mitochondrial dysfunction, disruption of calcium and thyroid hormone homeostasis, and modulation of gut microbiota. (Some cartoon components were derived from Biorender (https://www.biorender.com)).
Mitochondrial dysfunction: Multiple researchers have elucidated that PBDEs, especially PBDE-47, cause neurotoxicity by targeting mitochondrial dysfunction. PBDE-47 disrupts mitochondrial biogenesis by inhibiting the miR-128-3p/PGC-1α pathway, reducing mitochondrial DNA, ATP levels, and translation products in rat hippocampus and PC12 cells, leading to significant mitochondrial dysfunction and neuronal damage [31]. Subsequent investigations reveal that neonatal exposure to PBDE-47 leads to learning and memory deficits in adult rats, accompanied by mitochondrial abnormalities and apoptosis in the striatum. PBDE-47 blocks PINK1/Parkin-mediated mitophagy, causing defective mitochondria to accumulate, exacerbates mitochondrial dysfunction and results in neuronal death by via the activation of AMPK/ULK1 pathway [32]. PBDEs also induce neurotoxicity by activating the mitochondrial apoptosis pathway. This is demonstrated by PBDE-47 disrupting calcium ion homeostasis, leading to mitochondrial dysfunction and apoptosis in neurons [33].
Disrupting calcium homeostasis: PBDEs, particularly tetra- and penta-BDEs, significantly influence neuronal signaling, chiefly by inhibiting neurotransmitter uptake within synaptosomes and vesicles and disrupting Calcium (Ca2+) homeostasis [34]. Research indicates that BDE-47 and its metabolite, 6-OHBDE- 47, enhance vesicular catecholamine release via exocytosis in PC12 pheochromocytoma cells and significantly elevate intracellular Ca2+ concentrations, primarily through calcium release from the endoplasmic reticulum and mitochondria [35]. DE-71 affected the cholinergic system and locomotor activity by disrupting zebrafish larvae calcium homeostasis [33]. DE-71, BDE-47, and BDE-99 have been observed to inhibit calcium uptake in rat neural tissues, and BDE-47 interferes with the calcium ion (Ca2+) homeostasis in human neuroblastoma SHSY5Y cells and hippocampal neurons of rats inducing apoptosis [36,37] suggesting that PBDEs may indirectly affect cellular functions by altering Ca2+ regulatory mechanisms.
Alterations of thyroid hormone homeostasis: Thyroid hormones are essential for brain development, hypothyroidism linked to numerous neuroanatomical and behavioral abnormalities. Numerous studies have elucidated that PBDEs can disrupt thyroid homeostasis, which is linked to neurotoxicity [1,2]. Epidemiological studies have shown exposure to PBDEs associated with lower TSH during pregnancy, positive associations were found between prenatal PBDEs concentrations and children's neurobehavioral issues, including somatic complaints, withdrawal, sleep problems, and internalizing problems in girls, and somatic complaints and attention problems in boys, inverse associations were observed between the sum of BDE-47, -28, -99, -100, and -153 with thyroid hormones [12]. In a study using female Sprague-Dawley rats administered low doses of PBDE-47 orally disrupted thyroid follicle structure and enhanced thyroid tissue apoptosis. PBDE-47 induced Endoplasmic Reticulum (ER) stress and unfolded protein response, leading to autophagy defects and increased apoptosis, indicating that excessive ER stress and autophagy impairment are involved in maternal thyroid injury following perigestational PBDE-47 exposure [38]. Exposure of fetal human Neural Progenitor Cells (hNPCs) to BDE-47 and BDE-99 did not affect proliferation but reduced migration distance and differentiation into neurons and oligodendrocytes, simultaneous exposure with the Thyroid Hormone Receptor (THR) agonist triiodothyronine rescues these effects on migration and differentiation [39]. Human type II Iodothyronine Deiodinase (Dio2), which is crucial for regulating local cerebral Thyroid Hormone (TH) equilibrium by neuroglial cells, is significantly impacted by BDE209. The BDE209-induced degradation of Dio2 and the subsequent loss of its enzymatic activity in neuroglial cells cause TH disequilibrium and further neurotoxicity [40]. Neonatal rat hippocampal neuron-glia co-cultures exposed to BDE-47 or BDE-49 and treated with triiodothyronine (T3: 3-30 nM), NAC: (100 μM), or α-tocopherol (100 μM) showed that T3 and antioxidants effectively counteracted PBDE-induced axonal growth inhibition. T3 also prevented PBDE-induced ROS generation and mitochondrial metabolic disruptions, underscoring protective role in preserving axonal growth against PBDEs effects [41].
Neural cell differentiation and migration: Neural cells differentiation and migration are critical processes in neurodevelopment. Studies have shown that in vitro exposure to BDE-209 inhibits neurite outgrowth and the differentiation of neural stem cells into neurons in a dose-dependent manner, leading to a higher proportion of neural stem cells differentiating into glial cells [42]. Exposure of human neural progenitor cells to 1 μM BDE-47 or BDE-99 reduces the number of cells differentiating into neuron-like or oligodendrocyte-like cells and decreases their migration capacity [39]. Proteomics studies support these findings, showing that BDE-99 induces changes in the levels of cytoskeletal proteins involved in cellular migration, essential for cell migration [43,44]. Exposure of mice to BDE-209 or its hypobrominated metabolites (BDE-203 and BDE-206) during the critical period of rapid Brain Growth and Development (BGS) disrupts the levels of CaMKII, synaptophysin, BDNF, GAP-43, and tau, which are integral to brain development and function, contributing to processes such as neurite outgrowth and synaptic plasticity. This provides evidence that PBDE-209 can impair key components of normal brain maturation [45,46]. Proteomic analysis in humans with PBDEs exposure exhibited differential expression of 697 proteins, part of which involved cell structure [47]. In our study, we found that PBDE-209 inhibits the differentiation of hippocampal neural stem cells. This effect was reversed by IGF-1, which activated the PI3K/AKT and ERK1/2 signaling pathways, effectively countering the negative impact of PBDE-209 on cellular differentiation [48]. Research indicates that PBDEs, particularly PBDE-47 and PBDE-99, alters the expression of ionotropic glutamate receptors, which are critical for synaptic transmission and the regulation of cell differentiation in the central nervous system [49]. In conclusion, PBDEs impact neurodevelopment by inhibiting neurite outgrowth, reducing neural stem cell differentiation into neurons, impeding cell migration, altering cytoskeletal protein levels.
Effect on neurotransmitter systems: PBDEs disrupt the cholinergic system, affecting neurotransmitters by targeting nicotinic and muscarinic receptors crucial for learning and behavior. Exposure to PBDE-99 causing a decreasing density of cholinergic nicotinic receptors and muscarinic cholinergic receptors in hippocampus [50]. Additionally, research showed that binary mixtures 6-OH-PBDE-47 and PCB-47 could enhance inhibitory GABA (A)-related signals while suppressing excitatory α (4), β (2) nACh-mediated signals [51]. Following exposure to the PBDE mixture DE-71, changes in the GABAergic and glutamatergic systems in the frontal cortex were observed, revealing alterations in key proteins such as GAD67, vGAT, vGlut, and the GABA(A) 2α receptor subunit [52]. Moreover,DE-71 disrupts the nigrostriatal dopamine system, causing dopaminergic cell death, reduced striatal dopamine, impaired dopamine handling, and significant locomotor deficits in mice, indicating PBDEs as potential risk factors for Parkinson’s disease and other neurological disorders [53]. This highlights the impact of early developmental exposure to PBDEs on the Neurotransmitter Systems, which can subsequently affect cognitive functions and behavioral responses.
Others: The mechanisms of brominated diphenyl ether neurotoxicity are intricate, and there are several other perspectives that are also meaningful and warrant attention. A recent study explored the impact of developmental exposure to PBDE-47 on gut microbiota and serum metabolites, linking these changes to neurobehavioral effects in adult rats. Gestational and lactational exposure to PBDE-47 resulted in hyperactivity and anxiety-like behaviors. 16S rRNA sequencing revealed significant changes in gut microbiota composition, with decreased genera Ruminococcaceae and Moraxella, and increased Streptococcaceae, Deferribacteraceae, Escherichia-Shigella, Pseudomonas, and Peptococcus [54]. Metabolomic analysis identified shifts in serum metabolites related to amino acid, carbohydrate, nucleotide, xenobiotic, and lipid metabolism. These disruptions were correlated with neurobehavioral abnormalities, suggesting that gut microbiota dysbiosis and serum metabolite alterations mediate the neurobehavioral impairments induced by early-life PBDE-47 exposure [54] this research provides a novel perspective on understanding the mechanisms of PBDE-induced neurotoxicity, but further research is needed to determine whether adjusting the gut microbiota can alleviate toxicity.
A plethora of cognitive neuroscience, neurobehavioral science, and biological research clearly indicate the neurotoxic potential of PBDEs, PBDEs exerting their neurotoxic effects through mechanisms such as oxidative stress, mitochondrial dysfunction, autophagy, disruption of calcium homeostasis, alteration of thyroid hormone homeostasis, neurotransmitter systems, and interference with neural differentiation and migration, even impact on gut microbiota. Neuronal cells are susceptible to oxidative stress, which results in subsequent damage to DNA, proteins, and membrane lipids. The neurotoxicity of PBDEs is characterized by oxidative stress and related cell death, features similar to those observed in ferroptosis [30]. Caspase-3, a critical effector protease in cell death, and DJ-1, a product of the PARK7 gene, both play pivotal roles in the neurotoxicity induced by oxidative stress [55]. Benzyloxycarbonyl-Asp-Glu-Val-Aspfluoromethyl ketone (Z-DEVD-fmk), a specific caspase-3 inhibitor, and NAC, a thiol-containing antioxidant, have been demonstrated as neuroprotective compounds. Adeno-Associated Viral (AAV) vectors, recognized for their efficacy and safety, have been increasingly utilized in gene therapy for treating neurological disorders. NAC, along with ferroptosis inhibitors such as ferrostatin-1-a lipid reactive oxygen species scavenger-and the iron chelator deferoxamine mesylate, has been shown to mitigate the neurotoxicity induced by PBDE-47. Furthermore, the protein DJ-1 is a potential target for gene therapy using AAV vectors to counteract the neurotoxic effects of PBDE-47 [30]. Exposure to PBDE-47 in human neuroblastoma SH-SY5Y cells triggers increased autophagic activity, characterized by the formation of double-membraned ultrastructure, an increase in MDC-positive cells, and elevated levels of autophagy-related proteins LC3-II, Beclin1, and P62. The use of inhibitors such as NAC and autophagic inhibitor 3-Methyladenine (3-MA) enhances cell viability, indicating their potential neuroprotective effects by mitigating the autophagy-induced cell death, which is likely mediated by oxidative stress [25]. Autophagy impairment leads to apoptosis and cell death, with wortmannin reversing this effect in PBDE-47 cases [27]. Additionally, melatonin improves PBDE-47-induced neurotoxicity by preventing neuronal apoptosis and loss, restoring mitophagic activity and mitochondrial function via the AMPK/mitophagy axis, 32 therefore, both wortmannin and melatonin act as potential therapeutic compounds in alleviating neurotoxicity. As mentioned before PBDE-47 impairs mitochondrial biogenesis by inhibiting the miR-128-3p/PGC-1α axis resulting in mitochondrial dysfunction, 31 and further research showed the activation of the PGC-1α/ERRα axis through the compound ZLN005 restores normal mitochondrial function to mitigate neurotoxicity [56]. In vitro investigations with PC12 cells revealed that PBDE-47 disrupts mitochondrial dynamics, but the chemical promoter M1 or adenovirus-mediated overexpression of Mitofusin 2 (Mfn2) effectively mitigated these effects, improves mitochondrial function, prevents apoptosis, and enhances neuronal survival [24]. NMDA receptor plays an important role in neuronal cell death by causing an excessive influx of calcium, which triggers a series of potentially neurotoxic events. MK801 is known to be a potent and selective noncompetitive antagonist of the NMDA receptor, acting as an open-channel blocker at the NMDA receptor-operated ion channel. The introduction of the NMDA receptor blocker (MK801, 3lM) served to protect against an increase in calcium entry and significantly reduced cell apoptosis induced by DE-71, suggesting the involvement of the NMDA receptor [57]. It has been demonstrating that DE-71 impairs locomotor activity and cholinergic neurotransmission in zebrafish larvae through disruption of calcium homeostasis, this effect can be mitigated by the calcium channel agonist ( ± )-BAY K8644, as evidenced by significant increases in calcium concentrations, ACh contents, and locomotor activity during light-dark transition stimulation compared to exposure to the same concentrations of DE-71 alone [33], extracellular Ca2+ chelator (EGTA) showed similar effects [36], further research to explore if other calcium channel regulators produce similar effects is needed. Epidemiological studies and biological research proved that neurotoxicity of PBDEs exposure is linked to thyroid hormone disruption [12,38]. Triiodothyronine (T3) protects axonal growth by inhibiting PBDE-induced ROS [41]. The Insulin-like Growth Factor (IGF) system is crucial for regulating growth, particularly during fetal development, and it plays a central role in maintaining metabolic homeostasis. IGF-1 has demonstrated the capacity to reduce PBDE-induced apoptosis while promoting the proliferation and differentiation of neural stem cells in vitro experiments [48]. Additionally, it inhibits oxidative stress, prevents apoptotic cell death, and enhances neurite outgrowth and cell viability in DRG neurons [58]. Tyrosinase can regulate dopamine synthesis, and studies show that using tyrosinase inhibitors such as Phenylthiourea (PTU) can increase dopamine levels, significantly alleviating the hyperactive behavior of zebrafish embryos induced by PBDEs. This effect suggests that modulating tyrosinase activity holds potential as a therapeutic target for preventing and treating neurotoxicity caused by PBDEs [59]. These therapeutic approaches are potential for preventing the damaging effects of PBDEs exposure, particularly during important developmental stages.
The neurotoxic mechanisms of PBDEs are complex and intertwined. While pharmacological interventions targeting these key mechanisms have demonstrated potential neuroprotective effects, comprehensive research and clinical trials are essential to confirm their effectiveness and safety in addressing PBDEinduced neurotoxicity.
The author declares no conflict of interest.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Yang YX, Tan WH, Wen SW, Tang JQ, Zhang JJ, Zeng ML, et al. (2024). A Mini View of Polybrominated Diphenyl Ethers (PBDES) Developmental Neurotoxicity and Mechanism. J Clin Toxicol. 14:571.
Received: 24-Jun-2024, Manuscript No. JCT-24-33537; Editor assigned: 27-Jun-2024, Pre QC No. JCT-24-33537 (PQ); Reviewed: 11-Jul-2024, QC No. JCT-24-33537; Revised: 18-Jul-2024, Manuscript No. JCT-24-33537 (R); Published: 25-Jul-2024 , DOI: 10.35248/2161-0495.24.14.571
Copyright: © 2024 Yang YX, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.