
Journal of Clinical Trials
Open Access
ISSN: 2167-0870

ISSN: 2167-0870
Research Article - (2024)Volume 14, Issue 6
Introduction: Pancreatic Adenocarcinoma (PDAC) is one of the most lethal malignancies, with a dire need for new treatments. Locally Advanced Pancreatic Cancer (LAPC) often presents with limited therapeutic options, with median overall survival of only 6 to 11 months. Immunotherapy (IO) has shown promising results in various solid and hematological malignancies, but it is less effective in PDAC because of its immunosuppressive Tumor Microenvironment (TME) and cold tumor phenotype. This study explores the efficacy of Irreversible Electroporation (IRE) combined with IO in altering the TME, following standard-of-care Chemotherapy (CHT) and Radiation Therapy (RT), to enhance treatment response in LAPC.
Methods: This is a nonrandomized, single-arm, Phase 1 study for adult patients with histologically confirmed LAPC. The treatment involves a nonconcurrent combination of CHT, RT, IRE, and IO with pembrolizumab. The study assesses the safety, tolerability, and preliminary efficacy of this combined sequential therapy. Biopsies taken at the time of IRE and after IO, along with blood and serum samples taken regularly throughout therapy, will be used to assess changes in therapeutic responses in the TME and to identify both immunologic and tumor markers.
Discussion: The study hypothesizes that using a multistrike strategy will induce permanent alterations within the PDAC TME, turning it from cold to hot via RT and IRE to enhance the responsiveness to IO. This approach is based on evolutionary dynamics models, drawing from Anthropocene extinction events. If successful, this trial can not only improve LAPC outcomes but also contribute to the understanding of the biology of LAPC, potentially leading to the development of personalized therapies for LAPC. If successful, results from this study will lead to a Phase 2 randomized trial to further evaluate the efficacy of this treatment approach and contribute to the identification of novel biomarkers for personalized treatment strategies.
Pancreatic adenocarcinoma; Locally advanced pancreatic cancer; Irreversible electroporation; Immunotherapy; Radiation therapy; Chemotherapy; Evolutionary oncology; Genomic; Clinical trial
Pancreatic Ductal Adenocarcinoma (PDAC) is a highly lethal malignancy with limited therapeutic options, particularly for patients with locally advanced disease [1]. Novels therapies are urgently needed because pancreatic cancer is projected to become the second leading cause of cancer-related deaths in the United States by 2030 [2].
Despite advances in CHT, RT and Chemoradiation Therapy (CRT), patients with unresectable LAPC have shown poor median Overall Survival (OS) of 6 to 11 months in most prospective clinical trials. No Phase 3 prospective randomized clinical trial supports any specific treatment strategy for patients with LAPC. The current standard of care is based on consensus guidelines from leading authorities in oncology, such as the National Cancer Care Network (NCCN) [3]. The most common first-line treatment for LAPC is induction chemotherapy, usually followed by CRT, hypofractionated RT, or stereotactic body radiation therapy [4]. Induction CHT is usually Folinic acid, Fluorouracil, Irinotecan, and Oxaliplatin (FOLFIRINOX) or a modified FOLFIRINOX regimen [5]. For patients with marginal performance status, the CHT regimen is gemcitabine plus albumin-bound paclitaxel. The NCCN guidelines indicate 4 subsequent therapy options for patients with LAPC who have good performance status following first-line therapy: 1) Consider resection, if feasible, 2) Observe patients, 3) Continue systemic therapy and 4) Perform clinical trial [3].
Immunotherapy (IO) has revolutionized cancer treatment, demonstrating impressive results in various malignancies, such as melanoma and lung cancer [6,7]. However, pancreatic cancer has proven highly resistant to IO, primarily because of its immunosuppressive TME and cold tumor phenotype, characterized by a lack of immune-cell infiltration [8,9]. The TME in PDAC is highly complex and heterogeneous, consisting of a dense fibroinflammatory stroma with abundant extracellular matrix components, immunosuppressive cell populations, and limited vascularization [10,11]. This environment not only provides physical barriers to immune-cell infiltration but also actively suppresses antitumor immune responses through various mechanisms, such as recruiting regulatory T cells and myeloid-derived suppressor cells, secreting immunosuppressive cytokines, and upregulating immune checkpoint molecules [12,13]. In addition to the dense extracellular matrix and immunosuppressive cell populations, other factors contributing to the complexity of the TME include hypoxia and nutrient deprivation and the presence of various signaling molecules that can promote tumor growth and immune evasion [14,15]. These factors collectively contribute to forming a highly immunosuppressive TME that protects pancreatic cancer cells from immune-mediated destruction.
Several tumor-directed therapies have been developed to target the immunosuppressive TME and improve the efficacy of IO in pancreatic cancer. These approaches include RT, IRE and ultrasound- based modalities, each with its own challenges and limitations [16-18]. Locally ablative therapies, like IRE and stereotactic body RT, have been proposed to overcome these challenges by disrupting the stromal matrix and promoting immune-cell infiltration, effectively converting cold tumors into hot ones [19,20].
IRE uses high-voltage, short, direct-current electrical pulses to produce an electric field that induces electroporation on cells and creates nanoscale defects (permanent pores) in cellular membranes, resulting in loss of homeostasis and subsequent cell death [21-23]. The preliminary reports of the experience of IRE in patients with unresectable LAPC show encouraging results [24,25]. A recent systemic review of IRE in LAPC included 15 studies involving 691 patients [26]. Eight of the 15 studies were retrospective single- center studies, and the remaining 7 were prospective single-center or multicenter studies. The induction treatment varied, with 70% receiving induction CHT and only 20% to 50% receiving radiation therapy; the median OS in the combined cohort varied from 10 to 27 months. A recently initiated pivotal Phase 3 trial is a randomized control trial of FOLFIRINOX alone vs. FOLFIRINOX plus IRE in LAPC (NCT#03899636) [27].
We hypothesize that outcomes for patients with LAPC can be improved by using IRE as a strategy to alter the TME of PDAC, so that the LAPC will be more responsive to systemic IO. This sequential therapy approach is supported by dynamic evolutionary extinction models, which suggest that the timing of a second strike is a critical factor in inducing a lasting change within the TME [28]. This strategy uses the theoretical framework of evolutionary dynamics, using an Anthropocene extinction model. We predict that the IRE will alter the cold PDAC TME to hot, thereby making it more responsive to checkpoint inhibitor therapy. By administering IRE and Immune Checkpoint Inhibitor (ICI) therapy after first-line therapy (i.e., CHT followed by RT), we aim to target residual tumor cells that have survived initial treatment, potentially increasing the likelihood of a durable response.
This strategy uses the theoretical framework of evolutionary dynamics, with an Anthropocene extinction model. Anthropocene extinction events demonstrate that populations often follow one of 2 patterns after experiencing an initial insult. These populations enter what is referred to by academics as an “extinction vortex.” They then either continue diminishing to a point when they cannot recover and face eventual extinction, or they rebound because they are inherently resistant (or develop resistance) against the initial insult. Thus, a window exists after an initial insult (first strike) during which a second intervention or another insult (second strike) may be used to push a population to complete eradication, ergo, complete extinction. However, for the second strike to be effective, it often cannot rely upon the same strategy as the first strike, because of selection pressure. Instead, an alternative strategy that can cause an entirely different form of evolutionary pressure must be applied. This concept can be applied to treating cancer and to the dynamics of adjuvant therapy. Therefore, in this clinical trial, we propose a novel multimodal strategy informed by dynamic evolutionary extinction models of first-line CHT and RT followed by sequentially administered IRE and IO with pembrolizumab for patients with LAPC who have good performance status and are not candidates for surgical resection.
Ethics approval
The study is approved by the Institutional Review Board (IRB) of H. Lee Moffitt Cancer Center and Research Institute (MCC) (MCC Study No. MCC# 22325, Advarra IRB #Pro00075545). Informed consent will be obtained from the participants and/or legal guardians. The trial is registered at the US NIH (ClinicalTrials.gov) as #NCT06378047. The current protocol is version 3.0, January 25, 2024.
Study design
This is a nonrandomized, single-arm, single-institution, Phase 1 study of adult patients with LAPC. MCC is responsible for the coordination and trial management, and quality assurance including reporting, monitoring, and database management. To be eligible for this study, patients must first have registered and been consented to the TCC, which is the MCC Institutional Biobanking protocol. The TCC provides systematic consent, biospecimen collection, and patient-survival follow-up. Specimens from both TCC and this study will be used in future correlative studies to analyze the study’s exploratory endpoints. Consent and enrollment into this study begins after completion of SoC CHT and RT. SoC CHT must have consisted of at least six 14-day cycles over 12 weeks (± 7 days) of FOLFIRINOX, and standard imaging after chemotherapy. The latter may have occurred on the last day of chemotherapy but must have occurred before RT. RT must have consisted of Ablative Stereotactic Magnetic Resonance Image- Guided Adaptive RT (A-SMART) dosed at 10 Gy per fraction for a total of 50 Gy (isotoxic approach), delivered daily (Monday–Friday, with breaks for weekends and holidays), have occurred 7 to 21 days after the last day of SoC CHT, along with imaging after RT. The latter may have occurred within 4 weeks (± 7 days) after RT. IRE will occur on day 0, along with blood banking and tumor- tissue banking. IO will occur on day 5 (± 7 days), during which participants will be administered pembrolizumab (IO) as a single 200 mg dose via a 30 min intravenous infusion. Blood banking will again occur on the day of IO (before administration) and within 7 days following pembrolizumab administration. A biopsy after IO will also be an option, on the day of the latter blood banking. Participants will have their After Treatment Visit on day 36, with End-of-Study Visit on day 95. The study schema is shown in Figure 1.
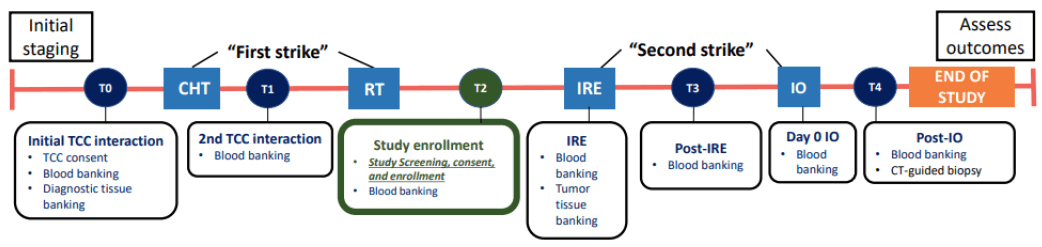
Figure 1: Clinical trial schema. Note: CHT: Chemotherapy; IO: Immunotherapy; IRE: Irreversible Electroporation; RT: Radiation Therapy; TCC: Total Cancer Care.
This Phase 1 study is designed to test the treatment safety and preliminary efficacy of combining sequential therapy of CHT, RT, IRE, and IO. This is an open-label, single-arm study with no randomization. Three patients will first receive the combined sequential therapy of CHT, RT, IRE, and IO. If none of the first 3 patients experience any Dose-Limiting Toxicities (DLT, defined as grade 3 or higher toxicities) definitively related to IRE and/or IO, we will conduct a Phase 2 of the study at a later date. If there is one DLT within the first 3 patients definitively related to IRE and/or IO, we will enroll an additional 3 patients. If no patients experience any DLT within the additional 3 patients definitively related to IRE and/or IO, we will conduct a Phase 2 of the study at a later date. Otherwise, we will not proceed to the Phase 2 study. If there is any fatal event (grade 5), the study will be closed with no further enrollment. Patients will undergo Standard-of- Care (SoC) active surveillance every 3 months (Q3Mo) after IO pembrolizumab for 24 months. SoC assessments include but are not limited to physical exam, vital signs, medical history, Eastern Cooperative Oncology Group status, hematology, chemistry, thyroid tests, blood collection, tumor markers, and imaging. All the patients will also be followed for vital status for up to at least 24 months after the IO dose for this Phase 1 study.
Eligibility criteria
All patients must provide informed consent before enrollment into the trial. Patients with a histologically confirmed diagnosis of localized LAPC and completion of Standard-of-Care Chemotherapy (SoC CHT) and RT can be included in this study. Patients can receive SoC CHT at an outside cancer center, but RT must be performed at the study institution. The main exclusion criteria are metastatic disease; gastrointestinal arteriovenous malformations; local gastrointestinal organ (eg, stomach, duodenum) invasion by tumor; and contraindications to ICI therapy. All eligibility criteria are shown in Table 1.
| Inclusion | Exclusion |
|---|---|
| 1. Histologically or cytologically confirmed pancreatic ductal adenocarcinoma meeting the American Joint Committee on Cancer, 8th ed. staging criteria of stage 3 disease. | 1. Clinical evidence of deep vein thrombosis or pulmonary embolism, prior history of cerebrovascular accident or history of transient ischemic attack within 12 months, or other known thromboembolism event present during the screening period. |
| 2. Tumor(s) is/are locally advanced and unresectable pursuant to NCCN guidelines. | 2. Clinically significant (i.e., active) cardiovascular disease: myocardial infarction (<6 months before enrollment), unstable angina, congestive heart failure (≥ New York Heart Association Classification Class II), serious cardiac arrhythmia requiring medication. |
| 3. Radiologically measurable disease per iRECIST, version 1.1 or based on exploratory surgery. | 3. Patients who have implanted cardiac pacemakers, defibrillators, or implanted devices with bare metal parts in the thoracic cavity, abdomen and/or retroperitoneum. |
| 4. Before TCC registration, participants must have no prior therapy for PDAC and fall under treatment NCCN pancreatic adenocarcinoma guides (version 1.2022) for locally advanced disease. Before Study Consent, participant must have had SoC first-line therapy consisting of 12 weeks of FOLFIRINOX (at least 6 cycles), followed by 50 Gy of A-SMART, delivered in five 10 Gy fractions. Participant must show no evidence of disease progression after first-line treatment, based on NCCN guidelines. | 4. Currently receiving treatment with medication that has a known risk to prolong the QT interval or to induce Torsades de Pointes and the treatment cannot be discontinued or switched to a different medication before starting study treatment. |
| 5. Age 18 to 74 years at diagnosis. | 5. Patient has other concurrent severe and/or uncontrolled medical conditions that would, in the investigator’s judgment, contraindicate patient participation in the clinical study. |
| 6. ECOG performance status 0-1. | 6. Contraindication to heparin as per institutional guidelines. |
| 7. The maximum axial and anterior to posterior tumor dimension must be ≤ 3.5 cm after first-strike SoC treatment. | 7. Another primary cancer within the last 3 years requiring systemic therapy. |
| 8. Participants must have adequate organ and marrow function as defined below, within 14 days (± 7 days) before IRE: Absolute neutrophil count should be ≥ 1000/μL; platelets should be ≥ 100 000/μL; hemoglobin should be ≥ 8 g/dL; total bilirubin should be ≤ 1.5 mg/dL; aspartate aminotransferase/alanine aminotransferase should be ≤ 3 times the institutional upper limit of normal or ≤ 5 times the upper limit of normal for subjects with documented metastatic disease to the liver; calculated creatinine clearance should be >30 mL/min; albumin should be ≥ 2.5 g/dL; coagulation PT time and international normalized ratio should be within normal limits (± 15%); partial thromboplastin time should be within normal limits (± 15%); and hemoglobin A1c should be ≤ 8%. | 8. Patient has had major surgery within 14 days before starting study treatment or has not recovered from major side effects. |
| 9. Life expectancy ≥3 months. | 9. Patient is currently receiving increasing or chronic treatment (>5 days) with corticosteroids or another immunosuppressive agent within 2 weeks of study participation or has an active autoimmune disease that might deteriorate when receiving an immuno-stimulatory agent. |
| 10. Accessible tumor for 2 on-study repeated tumor biopsies (following the initial prestudy biopsy that occurred, pursuant to TCC Consent). | 10. Patient is being treated at start of study treatment with any of the following drugs: a. Drugs known to be strong or moderate inhibitors or inducers of isoenzyme cytochrome P450 3A4 (CYP3A4) including herbal medications. b. Drugs with a known risk of inducing Torsades de Pointes. The patient must have discontinued strong inducers for at least 1 week and must have discontinued strong inhibitors before the treatment is initiated. Switching to a different medication before starting study treatment is allowed. |
| 11. Resolved acute effects of any prior therapy to baseline or grade ≤ 1 severity. | |
| 12. If a participant requires anticoagulation, treatment must be modified to enoxaparin. | |
| 13. At screening, all female participants of childbearing age will undergo a urine pregnancy test. If the urine test is positive or inconclusive, a serum test will be performed. Regardless of whether urine or serum, female participants of childbearing age must have a negative pregnancy test before enrollment to be eligible. | |
| 14. The study drug can harm the developing human fetus. For this reason and because the prestudy SoC chemotherapeutic agents used in this trial are known to be teratogenic, women of childbearing potential and men must agree to use adequate contraception (hormonal or barrier method of birth control; abstinence) before study entry and for the duration of study participation. In addition, women of childbearing potential must agree to continue using adequate contraception for 4 months after pembrolizumab administration. Should a woman become pregnant or suspect she is pregnant while she or her partner is participating in this study, she should inform her treating physician immediately. If a woman is breastfeeding, she should stop the study drug. | 11. Known infection with human immunodeficiency virus, hepatitis B, or hepatitis C. |
| 15. Patients who are willing and able to comply with scheduled visits, treatment plans, laboratory tests, biopsies when required and other procedures. | 12. Known prior severe hypersensitivity to investigational product, hyaluronidase, or any component in its formulations, including known severe hypersensitivity reactions to monoclonal antibodies (grade ≥ 3 as per Common Terminology Criteria for Adverse Events, version 5.0). |
| 16. The patient must be enrolled in MCC 14690 TCC Protocol for tissue and blood banking before full study enrollment in order to be eligible for this study and must have their initial biopsy tissue available. | 13. Patient has a medically documented poorly controlled psychiatric disorder(s), alcohol abuse, or drug abuse as defined according to the Diagnostic and Statistical Manual of Mental Disorders, 5th ed. |
| 17. Ability to understand and the willingness to sign a written informed consent document. | 14. Gastrointestinal arteriovenous malformations. |
| 18. Patients must be eligible for SoC NanoKnife therapy. | 15. Local gastrointestinal organ (e.g., stomach, duodenum) invasion by tumor. |
| 19. Human immunodeficiency virus infected participants must be receiving an effective anti-retroviral therapy for the past 6 months with undetectable viral load and normal CD4 count. | 16. Patient is unable to undergo MRI because of incompatible implanted device, body habitus and/or phobia unamenable to anxiolytic therapy. |
| 20. Participants with a history of chronic hepatitis B virus infection must have an undetectable hepatitis B viral load on suppressive therapy, if indicated. | 17. Patient needs concurrent bypass bile duct surgery or bypass gastric outlet obstruction surgery. |
| 21. Participants with a history of hepatitis C virus infection must have been treated and cured. For participants with hepatitis C virus infection who are currently on treatment, they must have an undetectable hepatitis C viral load. | 18. Clinical evidence of chronic obstructive pulmonary disease, emphysema, recurrent pneumonia within 6 months, or heavy tobacco use. |
| 19. Patient has any recent risk of active infection or poor wound healing. | |
| 20. Any unusual arterial or venous anatomy which increased risk for bleeding or formation of pseudoaneurysm. | |
| 21. Patients with a history of autoimmune disease are excluded. | |
| 22. Patients with prior interstitial lung disease are excluded. | |
| 23. Patients who have had any live vaccines within 30 days are excluded. | |
| 24. Patients with pancreas cancers with microsatellite instability-high or mismatch repair-deficient solid tumors are excluded. |
Abbreviations: AJCC: American Joint Committee on Cancer; ALT: Alanine Aminotransferase; AST: Aspartate Aminotransferase; ECOG: Eastern Cooperative Oncology Group; FOLFIRINOX: Folinic acid (leucovorin), Fluorouracil (5-FU), Irinotecan and Oxaliplatin; INR: International Normalized Ratio; IRE: Irreversible Electroporation; iRECIST: immune Response Evaluation Criteria in Solid Tumors; MRI: Magnetic Resonance Imaging; PT: Prothrombin Time; PTT: Partial Thromboplastin Time; SoC: Standard of Care; TCC: Total Cancer Care protocol, being the institutional research biobanking protocol.
Table 1: Inclusion and exclusion criteria.
Objectives
The primary objective of this study is to determine the safety and tolerability of combining sequential therapy of IRE and IO for patients with locally advanced unresectable pancreas cancer following first-line therapy (CHT and RT). Secondary objectives are related to disease control efficacy and include evaluation of Progression-Free Survival (PFS), OS, and objective response rate.
Exploratory objectives are concerned with the evaluation of the presence and degree of conversion from an immunosuppressive to an immunopermissive tumoral environment using comparative immunology biomarkers in blood and tumor tissue at time points between various interventions (e.g., single-cell RNA sequencing, neutrophil-lymphocyte ratio, T-Cell Receptor (TCR) repertoire, CD4+ T regulatory cells, and myeloid-derived suppressor cells). We will also evaluate the immunologic changes occurring in patients treated with combining sequential therapy of CHT, A-SMART, IRE, and IO and evaluate the effect on their pancreatic tumor markers.
Primary endpoint: Rate of high-grade (grade 3-5) adverse events based on the NCI Common Terminology Criteria for Adverse Events, version 5 related to treatment, measured from IRE through 90 days following IO.
Secondary endpoints: 1) PFS is defined as the time from the date of diagnosis to the first documented tumor progression or death by any cause, whichever occurs first, 2) OS is defined as the time from the date of diagnosis to the time of by any cause, 3) The objective response rate is defined as the proportion of patients with either a partial or complete response according to iRECIST, version 1.1 guidelines, measured from date of diagnosis [29].
Exploratory endpoints: 1) Immunologic markers on serum and blood (4-1BB, OX40, LAG3, ICOS, GITR, CTLA4, TIM-3 and PD-1), including the neutrophil-lymphocyte ratio, myeloid- derived suppressor cells (CD11b+Gr-1+), and TCR repertoire of CD8+ T cells (CD3+, CD8+), CD4+ T effector cells (T eff; CD3+, CD4+, FOXP3−) and CD4+ T regulatory cells (Tregs; CD3+, CD4+, FOXP3, +CD127−/lo), will be assessed according to the schema (Figure 1). We will discern the profile of tumor and TME cells by comparing immunologic cells from biopsies performed on the initial diagnostic pretreatment tumor specimen (TCC collection), intraoperative biopsy during IRE following the induction CHT and A-SMART (T2) (Figure 1), and finally, after IRE and IO therapy (T3) (Figure 1), 2) Tumor markers will be assessed and explored at the study time points (T1-T3) (Figure 1), as well as during follow-up. The tumor markers will include standard clinical markers such as Carbohydrate Antigen (CA)19-9, carcinoembryonic antigen, and CA-125 and novel markers in development such as circulating- tumor DNA (ctDNA) (T1-T3) (Figure 1), immunophenotyping (T1- T3) (Figure 1), cytokines, and TCR clonality, as well as radiomics.
First-strike therapy
Chemotherapy: All patients must have undergone SoC CHT of FOLFIRINOX for at least 6 cycles (12 weeks). It will be acceptable for patients to have received SoC CHT at an outside institution.
Radiation therapy: RT will be performed with an A-SMART approach on the Magnetic Resonance Imaging (MRI)-guided linear accelerator (MRL), ViewRay MRIdian (ViewRay) [30]. Treatment details at our institution have been previously reported [31-33]. A-SMART simulation will be performed without fiducial marker placement because of the direct tumor visualization provided by the ViewRay MRIdian system, obviating the need for a surrogate marker. Simulation will be performed with the patient laying supine with arms at their side for patient comfort without immobilization and with Deep Inspiration Breath Hold (DIBH) for 25 sec to obtain a 3-dimensional magnetic resonance image and a representative sagittal slice where the primary tumor is identified. MRIdian uses the MRI balanced steady-state free precession sequence (TrueFISP). The patient will be subsequently marked at the laser sites and taken to the Computed Tomography (CT) simulator. The patient is then placed in an identical supine position and undergoes a DIBH with and without intravenous and oral contrast. Target and Organ-at-Risk (OAR) contours are performed on the radiotherapy TrueFISP scan. The CT scan will then be deformably registered to the TrueFISP scan for predictive dose calculation. The MRIdian system uses a step-and-shoot intensity-modulated (RT) treatment delivery technique. Intensity-modulated RT plans will be generated with Monte Carlo dose calculation and magnetic field corrections.
Gross target volume and tumor-vessel interface are defined as gross tumor within pancreas as seen on diagnostic imaging and simulation CT or MR scans. This volume will be expanded by 3 mm to create the nominal Planning Target Volume (PTV). The PTV is then isotropically expanded by 3 cm to create an OAR evaluation structure, within which the OAR will be recontoured daily. OARs that require contours include the stomach, duodenum, small bowel, large bowel, kidneys, liver, and spinal cord. OARs that may trigger adaptation, including the duodenum, stomach, and bowel, are combined into a single structure and expanded by 5 mm to create a planning organ-at-risk volume. This avoidance structure is then subtracted from the nominal PTV to generate a PTVopti structure that will be modified by the daily adaptation process. The densWater, densAir, and densOther structures must also be added before plan exportation to account for daily density changes. PTV prescriptions will be 50 Gy delivered in 5 fractions in an isotoxic approach.
Before treatment delivery, the base plan will be used to determine the predicted dose distribution on the anatomy of the day. The new target and OAR metrics achieved by the initial plan on the daily anatomy are then evaluated to see if violations occur (Table 2). Online adaptation will be triggered if there was insufficient PTV coverage or if the critical OAR dose exceeded the predetermined allowed limits. Real-time tracking on a sagittal scan every 250 ms is performed with automatic gating (beam pause if target moves >5% outside of prespecified region). DIBH is used during treatment to optimize duty-cycle efficiency.
Second-strike therapy
| OAR | Objective |
|---|---|
| Bowel | 39.5 Gy max dose, <25 Gy mean |
| Stomach and duodenum | 38 Gy max dose, <38 Gy mean |
| Stomach, duodenum and bowel | V32 Gy cc ≤ 2 cc |
| Stomach, duodenum and bowel | V35 Gy cc ≤ 0.5 cc |
| Kidneys (right and left) | Mean <10 Gy |
| Spinal cord | 20 Gy max dose |
| Critical constraints triggering online adaptation | |
| Stomach, duodenum and bowel | Point dose max ≥ 39.5 Gy |
| Stomach, duodenum and bowel | Max 0.5 cc ≥ 35 Gy |
Note: *The hottest voxel is 10 Gy in 30% sub-volume that receives the lowest overall dose.
Abbreviations: A-SMART: Ablative Stereotactic Magnetic Resonance Imaging-Guided Adaptive Radiation Therapy; LAPC: Locally Advanced Pancreatic Cancer; OAR: Organs at Risk.
Table 2: Dose Constraints for 5 Fraction A-SMART for LAPC.
Irreversible electroporation: The study intervention of IRE is a relatively new technology that is a potentially ideal solution for ablating unresectable LAPC because IRE does not induce thermal tissue damage, thus avoiding injury to blood vessels and pancreatic and biliary ducts [21,22,34]. In this clinical study of IRE ablation using NanoKnife (AngioDynamics), we will perform the procedure according to the established standards and manufacturer’s instructions. Cardiac monitoring/synchronization will be used in accordance with the NanoKnife instructions. The technical specifications for use are described in Table 3. The maximum electroporation voltage, IRE pulsing scheme, and number of electrodes will depend upon the patient’s specific tumor characteristics but will always be done according to the manufacturer’s instructions.
| Component | Description |
|---|---|
| Number of probe outputs | 1-6 |
| Number of pulses* | 10-100 |
| Pulse amplitude | 500 to 3000 V |
| Pulse length | 20-100 µs |
| Pulse interval, unsync | 90 PPM, 670 ms/3.5 s every 10th pulse |
| Pulse interval, sync | Electrocardiogram, interval varies depending on heart rate |
| Maximum energy per pulse (nominal) | 15 J |
| Energy storage** | 100 µF minimum |
| Pulse amplitude precision | ± 5% |
| Pulse length precision | ± 2 µs or 2% (whichever is larger) |
| Maximum current | 50 A |
Note: *Number of pulses for each pair of electrodes; **Between recharges.
Abbreviations: ECG: Electrocardiogram; PPM: Pulse Per Minute.
Table 3: Irreversible electroporation treatment parameters.
Immune checkpoint inhibitor therapy: Patients will be administered pembrolizumab as a single 200 mg dose via a 30 min intravenous infusion, administered within approximately 1 week after IRE.
Radiological and pathological treatment evaluation: In accordance with NCCN guidelines, imaging will include CT of the chest and abdomen/pelvis pancreas. MRI and/or endoscopic ultrasound will be included as needed. However, it is expected that all second- strike imaging (i.e., after IRE and IO imaging), will be performed via CT. Imaging after IRE will occur 1 week (± 3 days) after IRE, on the same day as IO treatment but before the IO treatment on that day. Imaging after IO will occur 4 weeks (± 14 days) after the single IO dose (or 3 months (± 14 days after the last previously performed imaging)). Imaging after IO may occur within 1 week before the first follow-up visit, but if imaging occurs on the same day as the end-of-study visit, then it needs to be completed before the appointment with enough time for a diagnostic radiology read. All second-strike imaging (i.e., imaging after IRE and IO) should be performed via CT.
Core needle biopsies will be performed at the time of IRE and at 4 weeks after IO. The biopsy sample from the time of IRE will be used to assess the pathological response to first-strike therapy; the sample from after IO will be used to assess the response to second- strike therapy. The treatment response will be assessed according to the tumor-regression grading system of the College of American Pathologists.
Concomitant therapy: Using concomitantly prescribed drugs is allowed during this study. Participants may also take over-the- counter medication as needed. However, steroid medications or any herbal supplements containing steroids are prohibited.
Follow-up and adverse-event assessment: Adverse Events (AE) and Serious Adverse Events (SAE) assessment will occur during all clinical visits after time of enrollment. Collection will continue through 90 days (AE) and 30 days (SAE) following the end-of-study treatment (i.e., the single pembrolizumab IO dose). Survival will be assessed up to 24 months after the single pembrolizumab IO dose.
Follow-ups after IO imaging will occur every 3 months. Grading of AEs will be documented based on the Common Terminology Criteria for Adverse Events, version 5.0. SAEs will be those that result in death; a life-threatening AE; inpatient hospitalization or prolongation of existing hospitalization; a persistent or significant incapacity or substantial disruption of the ability to conduct normal life functions; or a congenital anomaly or birth defect. Any medical condition that is present when the participant is screened will be considered baseline and not reported as an AE. Participants removed from study for unacceptable AEs will be followed until resolution or stabilization of the AE.
Statistical considerations: Data will be summarized overall using descriptive statistics. For example, continuous data will be summarized with the number of patients (n), mean, median, minimum, maximum, standard deviation, coefficient of variation, and geometric mean (where applicable). Categorical data will be summarized using frequency counts and percentages. The occurrence of DLT will be summarized by frequencies and percentages. Response rates and 95% confidence intervals will be calculated using Wilson’s method for binomial probability distributions. OS and PFS will be estimated using the Kaplan– Meier method. If estimable, median OS and PFS with 95% confidence intervals will also be determined. Objective response will be summarized descriptively using frequencies and percentages.
As the Phase 1 trial is currently ongoing, specific results regarding the safety and efficacy of the sequential combination therapy involving CHT, RT, IRE, and pembrolizumab in patients with LAPC have not yet been fully analyzed or reported. However, the trial is designed to assess several key endpoints, including treatment tolerability, preliminary efficacy, and changes in the TME.
The study aims to collect data on adverse events and responses to treatment through various assessments, including imaging studies, biopsy evaluations, and biomarker analyses. Initial observations suggest that the combination therapy may have the potential to enhance immune activation and induce significant alterations within the TME, although comprehensive results will be available upon completion of the trial.
This novel treatment approach is based on an evolutionary dynamics model and is the first to adapt insights from Anthropocene extinction events into treating PDAC. This trial uses 2 local therapies (RT and IRE) to prime the TME for sequential IO. If successful, this trial would have implications beyond LAPC. We would be demonstrating the possibility of improving clinical outcomes by integrating evolutionary dynamic models into trial design.
Anthropocene extinction events demonstrate that populations often follow one of 2 patterns after experiencing an initial insult. These populations enter an extinction vortex, and then they are either diminished to a point where they cannot recover and face eventual extinction, or they rebound after having been selected for resistance against the initial insult. Intentional Anthropocene extinctions have taught us that a window exists after the initial insult (first strike) that allows for another insult (second strike) to push these populations to complete eradication after entering the extinction vortex [35,36]. However, for these second strikes to be effective, they often cannot rely upon the same strategies for continued demographic perturbation due to selection pressure [36]. Instead, an alternative strategy must be applied that can cause an entirely different form of evolutionary pressure. The strategy that would make a second strike effective often would not be effective as an initial strike because it relies upon the unique ecological system generated by the first strike. The temporal ecological disruption caused by the first strike to generate the extinct vortex enables the second strike to push the remaining populace to inevitable extinction, thus highlighting the importance of timing in a successful second strike. These lessons can be applied to the treatment of cancer and the dynamics of adjuvant therapy.
Initial cancer therapies typically result in significant reduction of the global tumor population [37]. Nearly all adjuvant therapy protocols focus on continued demographic perturbations. That is, they directly attack individual cancer cells to alter the proliferation and death rates of the population. Many times, this strategy will eventually result in a successful extinction vortex, thus eradicating the remaining cancer cells [38]. However, if resistance develops and manages to successfully produce a growing cohort before fading out, a surviving population could permit evolutionary rescue, such that the tumor recovers and proliferates, eventually forming clinical metastases [36-38]. The period of time when the tumor is in the extinction vortex represents an opportunity to add new treatments for accelerating the extinction process and for reducing the risk of evolutionary rescue. However, in cancer, these tumor populations often have subpopulations with varying resistance to adjuvant therapy. In addition, these subpopulations rely on direct cell kill and, consequently, they present risks selecting for resistance phenotypes [39]. If adjuvant therapy can produce a permanent ecological disruption, then this entirely different form of selection pressure is applied that can affect the entire remaining tumor population [36-38]. By using a second strike in LAPC that focuses on permanent ecological disruption to the entire TME, a more sustained response may be achievable. Specifically, the activation of coexisting immune cells by IO has the potential to induce these long-lasting changes in the TME caused by eradication of small or subclinical cancer cell populations. However, pancreatic adenocarcinoma is known to be a cold tumor, meaning that very few antitumor immune cells are able to penetrate into or around the tumor, thus minimizing the efficacy of immune checkpoint inhibitors [40]. Fortunately, both ablative RT and IRE have been shown to potentiate immune response after therapy. These therapies may be able to convert this tumor into a more immune-permissive state during the extinction vortex window and allow for more permanent ecological changes of the TME via IO.
Preclinical data suggest that radiation enhances the antigenicity of tumors [41-44]. An orthotopically implanted PDAC-tumor murine model demonstrated that radiation enhances T-cell responses to immunogenic tumor baseline responsiveness to single-agent Programmed Death-1 receptor (PD-1) blockade [45]. In addition to enhancing T-cell responses, radiation-induced immunogenic cell death also triggers negative feedback in the form of immunosuppressive myeloid cells; these cells infiltrate the irradiated tumor and surrounding tissues to support wound healing and tissue homeostasis. This immune reaction also affects hematopoiesis in the bone marrow, leading to systemic release of immune-suppressive myeloid cells into circulation [46]. Specifically, this was shown in a murine model of ablative RT in combination with IO [47]. Therefore, preclinical data suggest that ablative RT may offer a unique immunomodulatory priming effect before IRE.
Additionally, IRE has demonstrated potential to induce a more immunopermissive TME in PDAC for IO in both preclinical and clinical studies. IRE can induce transient softening of tumor stroma, increase microvascular density, and increase permeability as recently shown in an orthotopic murine PDAC model [48]. These effects reversed resistance to anti–PD-1 therapy, inducing CD8+ T-cell infiltration and immunogenic cell death of cancer cells more effectively than the combination of irradiation and anti- PD-1 therapy, with significantly prolonged survival. Additionally, an immunocompetent mouse model demonstrated that combining IRE with the intratumoral Toll-like receptor 7 agonist and systemic anti–PD-1 checkpoint blockade resulted in both regression of primary tumor and elimination of untreated concomitant distant tumors (abscopal effect) [49]. These results suggest that the systemic antitumor immune response triggered by IRE can be enhanced by stimulating the innate immune system as well as the adaptive immune system. Similarly, IRE of LAPC transiently alleviates immune suppression and creates a window for antitumor T-cell activation. Moreover, after IRE, the tumor-specific systemic T-cell response to the PDAC-associated antigen, Wilms tumor 1, was related to longer OS [50]. Clinical evidence has also demonstrated how IRE attenuated the effect of CD4+ regulatory T cells (Tregs) in 11 patients with LAPC between postoperative days 3 and 5 [51].
We report preclinical and clinical observations of IRE modulation of the immune environment to enhance the effectiveness of IO and the effectiveness of pembrolizumab as IO with engagement of the immune system. Further, these observations suggested the logical next step of investigating IRE combined with pembrolizumab in LAPC. A Phase 1b trial investigated the use of concurrent IO and IRE in LAPC, which demonstrated both feasibility and safety of combining these therapies [52]. In the context of this proposal, none of the previous or ongoing clinical trials aim to assess the role of sequential IRE with IO as part of a multistrike treatment approach following first-line CHT and RT. We anticipate that sequential use of CHT, RT, and IRE will alter the cold PDAC TME to hot, thereby making it more responsive to checkpoint inhibitor therapy with pembrolizumab.
Furthermore, we also aim to improve understanding of LAPC radiobiology. For this effort, we will use the diagnostic biopsy tissue, tissue taken during IRE, and tissue taken after IO to study the impact of the different therapies to better understand their influence upon TME, genomics, pathways activated, and the contribution to circulating-tumor DNA (ctDNA). Radiomic data will be collected from any imaging, including of diagnostic examination, restaging after therapy, and follow-up. These data will be analyzed in conjunction with blood, plasma, and tumor genomic data to identify potential radiomic and genomic biomarkers. These biomarkers have considerable potential to guide future studies to improve personalized RT for LAPC, such as improving patient selection and predicting patients at high-risk for treatment failure who may benefit from further adjuvant therapies.
Overall, this trial takes full advantage of current understanding within the fields of cancer biology, evolutionary oncology, radiation oncology, gastrointestinal oncology, and immuno-oncology, to push beyond the current treatment paradigm for LAPC. This trial represents an important step toward incorporating evolutionary oncology concepts by using sequential combinational therapies to purposefully induce an extinction vortex and subsequent extinction of cancer cells. If successful, we will build from this trial and open a randomized Phase 2 to determine the efficacy of the IO within this treatment paradigm. In addition, the insights gathered may also enable the identification of novel biomarkers that may refine current personalized strategies for LAPC patients.
While definitive results from this ongoing Phase 1 trial are pending, the design and objectives hold potential for advancing treatment strategies for LAPC. Should the trial demonstrate favorable safety and efficacy profiles, it will lead to the development of a Phase 2 randomized trial to further evaluate this innovative approach. Additionally, the insights gained may contribute to identifying novel biomarkers for personalized therapies, ultimately aiming to improve outcomes for patients facing this aggressive malignancy. The findings of this study could significantly enhance our understanding of LAPC treatment and lead to future therapeutic advancements.
Partial funding from AngioDynamics, Inc. and the Moffitt Foundation.
No author has any conflicts of interest pertaining to this work.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Bryant JM, Mo Q, Chin A, Hodul P, Denbo J, Anaya D, et al. (2024). A Multistrike Evolutionary Oncology Strategy for Locally Advanced Pancreatic Cancer: A Phase 1 Trial of Standard-of-Care Plus Irreversible Electroporation and Pembrolizumab Immunotherapy. J Clin Trials. 14:572.
Received: 27-Sep-2024, Manuscript No. JCTR-24-34318; Editor assigned: 30-Sep-2024, Pre QC No. JCTR-24-34318 (PQ); Reviewed: 14-Oct-2024, QC No. JCTR-24-34318; Revised: 21-Oct-2024, Manuscript No. JCTR-24-34318 (R); Published: 28-Oct-2024 , DOI: 10.35248/2167-0870.24.14.572
Copyright: © 2024 Bryant JM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.