
Immunotherapy: Open Access
Open Access
ISSN: 2471-9552

ISSN: 2471-9552
Research Article - (2021)Volume 7, Issue 5
Background: PD-L1 is expressed widely in the body. PD-1-PD-L1 interaction is known to drive T cell dysfunction, which can be blocked by anti-PD-1/PD-L1 antibodies. Researchers have shown that blocking PD-L1 in both the early and chronic stages of the disease may increase T cell activity. What’s more, we tried to find the improvement of PD-L1 on the body's immunity.
Methods: Nanobody binding to PD-L1 was prepared, and the PD-L1 nanobody was verified by SDS-PAGE and Western-Blot. Affinity detection of PD-L1 Nanobody and PD-L1 receptor was made by ELISA and flow cytometry. The cytotoxicity of the PD-L1 nanobody was tested by BHK-21, MDBK, and sheep kidney cells. The inhibitory effect on tumor model was verified. PD-L1 nanobody activated the macrophages was tested. Staphylococcus aureus was used to test the protective effect of the mice.
Results: PD-L1 nanobody was successfully made and had a high affinity with PD-L1 receptor. PD-L1 has no cytotoxicity to many cells. It could decrease the tumor in weight. We also find PD-L1 nanobody activated the macrophages and protected the mouse from the challenge of Staphylococcus aureus.
Conclusion: The PD-L1 nanobody improved the immunity of animals. It was verified that PD-L1 inhibited T cells may be always present in mice, and the activation of these cells improved the immunity and survival rate of mice.
PD-L1; Nanobody; Immune; Tumor; Staphylococcus aureus
Programmed cell death-1 (PD-1, Pdcd1), an immuno receptor belonging to the CD28/CTLA-4 family negatively regulates antigen receptor signaling by recruiting protein tyrosine phosphatase, SHP-2 upon interacting with either of two ligands, Programmed cell death ligand-1 (PD-L1) and ligand-2 (PD-L2) [1]. PD-1 is an important immunosuppressive receptor, a transmembrane type I glycoprotein [2]. It has been established that PD-1 is an inhibitory receptor up-regulated by activated T, B, and NK lymphocytes and that its ligand PD-L1 mediates a negative feedback of lymphocyte activation, contributing to the restoration of the steady state condition after acute immune responses [3]. Binding between T cell-intrinsic PD-1 and APC-intrinsic PD-L1 triggers inhibitory signaling to attenuate the T cell response [4]. PD-1 was expressed mainly on CD4(+) CD25(+) T cells [5]. Recent studies have revealed that membrane-bound PD-1 and PD-L1 also have soluble forms [6]. Beyond potent inhibitory effects on T cells, PD-1 also has a role in regulating B cell and monocyte responses [7]. PD-L1 is regulated by signaling pathways, transcription factors and epigenetic factors, such as the GSK3β/β-catenin pathway, P53 protein and EMT [8]. PD-L1 has been shown to negatively regulate immune responses via its interaction with PD-1 receptor [9]. T-cells are a type of lymphocyte (a subtype of white blood cells) that plays a central role in cell-mediated immunity [10]. Tregs might be involved in the treatment of PD-1/PD-L1 blockade and PD-1/PD-L1 axis could influence Treg differentiation and function [11]. The T-cell response is central in the adaptive immune-mediated elimination of pathogen-infected and/or cancer cells. This activated T-cell response can inflict an overwhelming degree of damage to the targeted cells, which in most instances leads to the control and elimination of foreign invaders [12]. Inhibitors of PD-1 signaling have revolutionized cancer therapy [13]. T cell exhaustion is often associated with inefficient control of persisting infections and cancers, but re-invigoration of exhausted T cells with inhibitory receptor blockade can promote improved immunity and disease outcome [14]. Current immunotherapies yield remarkable clinical outcomes by boosting the power of host immunity in cancer cell elimination and viral clearance [15]. An increasing amount of evidence supports the therapeutic potential of targeting exhausted T cells and T (SCM) cells [16].
Antibodies that block PD-L1 and PD-1 binding have been used for the prevention and therapy of human pathogenic diseases, but have not yet been evaluated for the treatment of infectious diseases of livestock [17]. Increased IFNγ signaling following anti-PD-L1 treatment can remodel the macrophage compartment to enhance T-cell responses [18]. Importantly, addition of antibodies against PD-1 or PD-L1 restored function in neutrophil, monocyte, T cells, and NK cells, underlining the impact of the PD-1: PD-L1 axis in sepsis-immune suppression [19]. PD-L1 cannot engage PD-1 to inhibit T cell activation when APCs express substantial amounts of CD80 [20]. The identification of B7-1 as an additional binding partner for PD-L1, together with the discovery of an inhibitory bidirectional interaction between PD-L1 and B7-1, reveals new ways that the B7:CD28 family regulates T cell activation and tolerance [21]. Researchers are developing effective immunotherapy to combat T cell exhaustion and re-energize T cells [22].
Nanobody (VHH) is a special variable region of heavy chain antibody, which exists in camels, sharks. The advantages of VHH, such as stable structure, small volume, strong penetrating ability, low production cost, large yield, and excellent affinity with antigen sites, make nanobodies the first choice for inhibitors [23]. In this study, PD-L1 nanobodies were prepared and verified that PD-L1 nanobodies can improve immunity.
Materials
Cells: Baby Hamster Syrian Kidney (BHK-21) cells, Madin Darby Bovine Kidney (MDBK) cells, Sheep kidney cells (kidney) and mouse macrophages were provided by Xinjiang Key Laboratory of High Incidence of Local and Ethnic Diseases, Shihezi University (Shihezi, China).
Materials: The IFN-γ (SEKM-0031), nitric oxide (NO) (BC1475), TMB color developing solution and IL-4 detection kits (SEKM0005) were purchased from Beijing Solarbio Science & Technology Co., Ltd. (Beijing, China). The MTS Cell Proliferation Colorimetric Assay Kit (Catalog#K300-500) was purchased from BioVision (Milpitas, CA, USA). Fetal bovine serum (FBS) was purchased from Gibco (USA). Mouse anti-His monoclonal IgG antibody and horseradish peroxidase (HRP) labeled rabbit anti-mouse IgG was purchased from Shenggong Bioengineering (Shanghai) Co., Ltd.
Methods
SDS-PAGE identification of PD-L1 protein and PD-L1 nanobody: SDS-PAGE was made by a 12% separation gel and 5% stacking gel; 8 μl of the sample was loaded on the stacking gel with the voltage adjusted to 80 V. Staining was performed using Coomassie Brilliant Blue staining solution.
WB identification of PD-L1 protein: PD-L1 protein was transferred to NC membrane and blocked overnight at 4°C using 5% PBSM. It was then washed 3 times with PBST. The 1000-fold diluted mouse Anti-PD-L1 (ABM4E54) to PD-L1, Anti-PD-L1 (ABM4E54) ab210931 antibody was added and incubated at 37°C for 1 hour. It was washed 3 times with PBST. A 2000-fold diluted Goat antimouse polyclonal antibody IgG-H&L (HRP) (abcam ab6789) was used. After washing 3 times with PBST, color development was performed using a DMB solution.
Affinity detection of PD-L1 Nanobody and PD-L1 receptor: The coating solution was used to dilute the PD-L1 protein to 100 ng per well, and it was added to a 96-well ELISA plate, and blocked with 5% PBSM overnight. After washing 3 times with PBST, the serial dilutions were added to the ELISA wells coated with PD-L1 protein. PD-L1 nanobody was added 50 µl per well in serial dilutions. After incubating for 2 hours of 37°C, the 96-well ELISA plate was washed for 3 times of PBST. The mouse anti-alpaca antibody was added in 2,000-fold diluted solution. After incubating at 37°C for 1 hour, the 96-well ELISA plate was washed for 3 times of PBST. After the rabbit anti-mouse HRP antibody with 10,000-fold diluted was incubated at 37°C for 1 hour. It was washed 3 times with PBST, and TMB color developing solution was added and incubated at 37°C for 15 minutes. The reaction was terminated with the stop solution and the OD450 was read.
Cytotoxicity of the PD-L1 Nanobody: BHK-21, MDBK, and sheep kidney cells were incubated in DMEM medium containing 10% FBS for 6 h at 37°C. The PD-L1 nanobodies were added in final concentrations of 5, 10, 20, and 40 μg/mL. Cells were then incubated at 37°C for 38 h. 20 μL/well of MTS reagent was added for 3 h at 37°C. The absorbance was measured at 492 nm after shaking.
Flow cytometry to detect the affinity of PD-L1 Nanobody and PD-L1 receptor: Nanobodies with 1 μg/mL was added 50 μL to a test tube for each stream. PBS was added 50 μL to the control tubes. The PC-3 cell suspension (about 106 cells) was added 50 μL to each tube and mixed gently. The tube was incubated in a refrigerator at 4°C for 60 minutes. After incubation, 2 mL PBS was added to each tube in the flow rate detection. Centrifuge was performed at 800 rpm at 4°C for 3 minutes, and the supernatant was discarded. Washing procedure was repeated three times. Add 100 μL of diluted anti-His tag antibody of each flow cytometry microporous tube or plate (diluted 1:100 with PBS). The samples were washed three times at 4°C. The cells were re suspended and tested.
Validation of humanized mouse tumor model: 20 female hPDL1- C57BL/6 Mice which were PD-L1 humanized and 7-week-old were inoculated subcutaneously with 1×106 MC38 cells. When the average tumor volume reached 100 mm3, mice with moderate tumor volume and weight were selected into the group, and they were divided into 2 experiments. In the group, 6 animals were enrolled in each group, and the administration was started on the day of grouping. The intra-peritoneal injection dose was 7 mg/kg, twice a week, 6 times in a row. Measure the diameter of the tumor with a vernier caliper, the calculation formula is V=0.5×Length×Width2. After tumor inoculation, monitoring included tumor growth, weight gain, and the effect of treatment on animal behavior. At the end of the experiment, the tumor-bearing mice were euthanized, the tumor masses were peeled off and weighed, and pictures were taken separately.
PD-L1 nanobody activated macrophages: Mouse macrophages were incubated in DMEM medium containing 10% FBS in 96-well plates at 37°C for 3 h. The phage was added 1 µg/mL. Cells were incubated for 24 h at 37°C. PBS and the PD-L1 nanobody were added in final concentrations of 5, 10, 20, and 40 μg/mL. Cells were incubated for 36 h at 37°C. The contol group was added to 100 μL extract, 50 μL reagent one, and 50 μL reagent two. The PD-L1 group and PBS group was added to 100 μL sample, 50 μL reagent one, and 50 μL reagent two. After 15 min agitation at room temperature, the absorbance was measured at 550 nm.
PD-L1 nanobody improve the mice immunity: Thirty-two BALB /C mice were injected with PD-L1 nanobody, each injected with 0.2 mL at a concentration of 0.5 mg/mL. Three days later, blood samples were collected, and serum samples were separated to detect cytokines IL-4, IFN-γ and NO. Staphylococcus aureus was injected with minimum lethal dose 1.9 ×109 (150 μl) cfu. A total of 16 mice were injected. Among them, 8 mice were injected in the PBS group and 8 mice in the PD-L1 group. The state of the mice was observed 24 hours later.
Data analysis: The results are expressed as mean ± standard deviation (SD). Statistical analysis was performed using Graph Pad Prism 8 software, and significant difference analysis was performed by Mann-Whitney U test (*=P<0.05, **=P <0.01).
Preparation and characterization of PD-L1 nanobodies
The target band of PD-L1 was consistent with the expected size (Figure 1A), which proved that the protein was successfully expressed and that PD-L1 was expressed in a soluble form. PD-L1 has 4 purified nanobodies (Figure 1B). The high specificity of PDL1 protein was demonstrated by WB (Figure 1C). The nanobody still has affinity for the PD-L1 receptor when diluted 3680 times (Figure 1D). Nanobodies have no cytotoxicity to mouse, sheep and cattle cells at different concentrations (Figure 1E). Phage was added to primary cells induced by mouse bone marrow stem cells. After adding the PD-L1 nanobodies and incubating for 24 hours, the PD-L1 nanobody concentration was positively correlated with the enhancement of NO (Figure 1F).
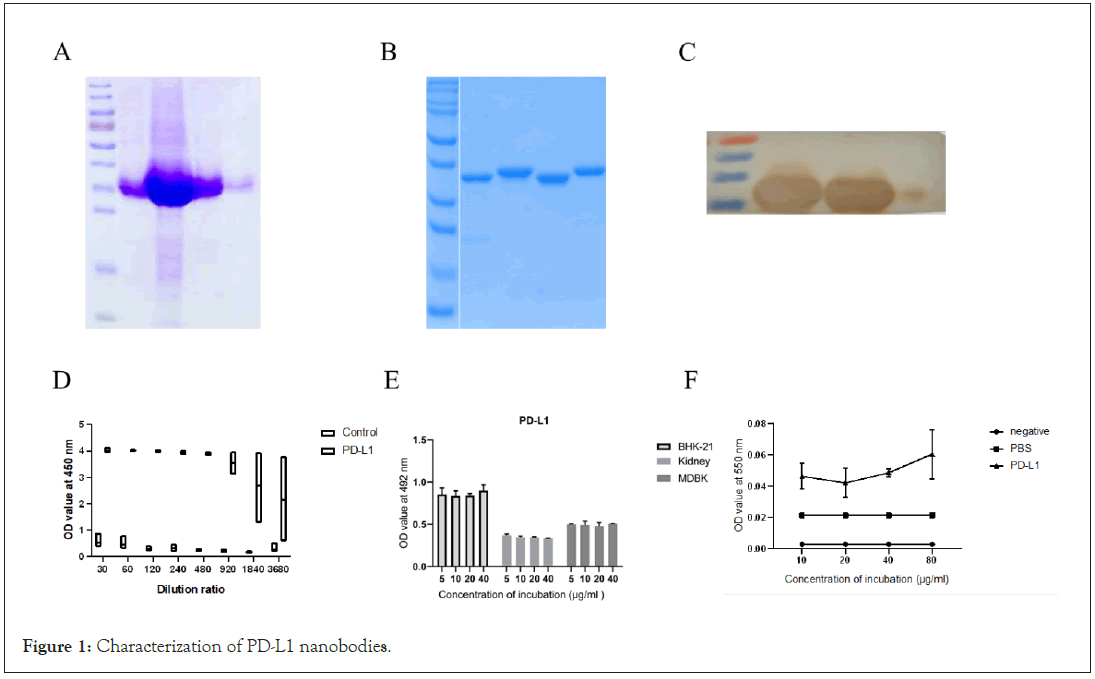
Figure 1: Characterization of PD-L1 nanobodies.
A: SDS-PAGE identification of PD-L1 protein. M is protein Marker, 1-5 are protein samples; B: SDS-PAGE identification of PD-L1 nanobodies. M is a protein molecule Marker, 1-4 is a different protein sample; C: Western-blot identification of PD-L1 protein. D: Affinity determination results of PD-L1 Nanobodies and PD-L1 receptor. Note: The abscissa is the gradient dilution factor, and the ordinate is the OD450 value. E: Cytotoxicity of PD-L1 Nanobodies. Note: The abscissa is treated with different concentrations of PD-L1 (μg/mL), and the ordinate is the OD value at 492 nm. BHK-21 is the BHK-21 cell experimental group. Kidney was the experimental group of sheep kidney cells, MDBK was the MDBK cell experimental group, and the data were expressed as mean ± SD. F: NO enhancement of PD-L1 in mouse primary cells. Note: Negative is a kit negative control; PBS is a negative control with PBS added, and PD-L1 is an experimental group with PD-L1 Nanobody added.
Flow cytometry results of PD-L1 Nanobody
PD-L1 Nanobody and MC-38 cells are shown in the Figure 2. The results of flow cytometry data show that the binding ability of PDL1 Nanobodies to PD-L1 is different. Among them, S3 and S6 Nanobodies have the strongest binding ability.
Figure 2: Flow cytometry of PD-L1 nanobody.
Note: A: Use MC-38 cells to express PD-L1 receptor. B-I: The flow cytometry results
Validation of humanized mouse tumor model
On the 21st day after administration, the average tumor volume in the control group was 3329.20 ± 1061.19 mm3. The tumor volume in the PD-L1 nanobody group was 2623.70 ± 327.25 mm3. The tumor inhibition rate was 22.10%. The average tumor weight in the control group was 3.5368 ± 0.7332 g. The average tumor weight in the PD-L1 nanobody group was 2.7634 ± 0.2830 g. The result of mouse tumor model is shown in Figure 3.
Figure 3: The result of mouse tumour model.
Test results of mice attacked by Staphylococcus aureus
PD-L1 Nanobody could protect animals when they were attacked by Staphylococcus aureus and enhanced the number of mice that survived. The result of mice attacked by Staphylococcus aureus is shown in Figure 4.
Figure 4: The result of mice attacked by Staphylococcus aureus.
Note: The data analysis was performed by Student's T test using EXCEL software, *<0.05 and **<0.005.
PD-1/PD-L1 blockade has an immune-regulatory activity which may synergize with the antiviral effect of IFN-alpha therapy [24]. The CD8 (+) T cell priming is directed essentially as a corroboration work between cells of innate immunity including dendritic cells (DCs) and natural killer (NK) cells with CD4 (+) T cells in adoptive immunity [25]. Cytotoxic CD8(+) T lymphocytes (CTL) efficiently control acute virus infections but can become exhausted when a chronic infection develops [26]. CD8 (+) HLA-DR (+) Treg-induced suppression on CD8 (+) responder T cells was abrogated by an anti- PD1 neutralizing antibody [27]. In cancer and chronic infection, this differentiation program is derailed, and antigen specific CD8 T cells differentiate to a hyporesponsive state generally referred to as T cell exhaustion [28]. At the same time, macrophages are more sensitive to antigens and can be used to detect the strength of the immune response. The co-inhibitory receptor PD-1, expressed on T cells, delivers negative signals when engaged by its ligand PD-L1, expressed on dendritic cells, Mϕ, and endothelial cells to attenuate T cell activation, effector functions, and survival [29]. PD-1 has recently been shown to be highly expressed on exhausted T cells during chronic viral infection, and blockade of PD-1 or PD-L1 can revive exhausted T cells, enabling them to proliferate and produce effector cytokines [30]. As PD-L1 are expressed widely in the body and affect the responses against self and foreign antigens, controlling PD-1/PD-L interactions enables the management of several immune-related diseases such as autoimmune disease, virus infection, and cancers [31]. PD-1-PD-L1 interaction is known to drive T cell dysfunction, which can be blocked by anti-PD-1/PDL1 antibodies [32]. PD-L1 blockade also resulted in increased in vivo NK cell persistence and retention of their cytotoxic phenotype [33]. The development of these small molecule inhibitors opens a new avenue for tumour immunotherapy based on PD-1/PD-L1 signaling pathway [34].
T cell exhaustion is a state of T cell dysfunction that arises during many chronic infections and cancer [35]. It is defined by poor effector function, sustained expression of inhibitory receptors and a transcriptional state distinct from that of functional effector or memory T cells [35]. Despite investigation of exhaustion, mostly about CD8 responses toward viral infections, recent studies have reported that chronic exposure to antigen may develop exhaustion in CD4+ T cells, B cells, and NK cells [36]. PD-1 and PD-L1 have attracted wide attention from researchers in the field of immunotherapy [37]. PD-L1 is an immune-inhibitory molecule that suppresses the activation of T cells [38]. The molecular pathways involved in T cell exhaustion remain poorly understood [39]. Reversing T-cell exhaustion is a promising immunotherapy for cancer that has yielded encouraging results [40].
There are many shortcomings in this study. For example, although the mice used for tumor models are humanized mice, the mice used for challenge protection are ordinary mice. Before and after the injection of PD-L1 Nanobody, the levels of IFN-γ, IL-4 and NO did not change, but they played a protective effect. It has not been further verified which cells produced the effect. The cells which have changed in the mouse needs to be further verified.
PD-L1 acts as a regulatory molecule on T cells and can inhibit the activity of T cells [41]. PD-1 also could be expressed on T cells, B cells and macrophages [42]. PD-L1 blockade together with CD4 T cell depletion effectively rescued deeply exhausted CD8 T cells and enhanced antiviral control during the late stage of chronic infection without any associated mortality [43]. Interaction with PD-L1 contributes to functional exhaustion of responding T cells and may limit immunopathology during infection [44]. Notably, the immune-regulatory molecule PD-L1 plays a determinant role in controlling/inhibiting activated T cells and thus maintains immune tolerance [45]. PD-1/PD-L1 inhibitory signal pathway has been verified to be involved in the establishment of persistent viral infections [46]. Viruses often subvert antiviral immune responses by taking advantage of inhibitory immune signalling [47]. Up regulation of PD-1 and its ligands PD-L1 and PD-L2 is observed during acute virus infection and after infection with persistent viruses including important human pathogens such as human immunodeficiency virus (HIV), hepatitis C virus (HCV), and hepatitis B virus (HBV) [48]. Dysfunctional virus-specific T cells are a hallmark of many chronic viral infections [49]. A (H1N1) pdm09 virus induced PD-L1 expression on human dendritic cells (DCs) and T cells, as well as PD-1 expression on T cells [50]. Regulatory T (Treg) cells act as terminators of T cell immuniy during acute phase of viral infection [51]. However, it has recently been shown that during the initial phase of infection virus-specific CD8+ T cells express high levels of PD-1, but are fully competent in producing cytokines and killing virus-infected target cells [52]. Moreover, microglial cells and astrocytes govern the activity of brain-infiltrating antiviral T-cells through up regulation of PDL1 expression [53]. Absence of PD-1 enhanced proliferation of T cells in adenovirus-infected livers and resulted in a rapid clearance of the virus [54]. T cell exhaustion can be partially or completely reversed by blocking inhibitory receptors such as PD-1 [35]. T-cell proliferative dysfunctionality could be reverted by PD-1/LAG-3 coblockade [55]. In addition, exhausted T cells during chronic viral infections could be revived by PD-L1 blockade [56]. Co-blockade of PD-L1 further enhanced T effector cell function, resulting in superior anti-viral and anti-tumour immunity over single target blockade [57].
These researchers have shown that blocking PD-L1 in both the early and chronic stages of the disease may increase T cell activity. The PD-L1 nanobody improved the immunity of animals. It was verified that PD-L1 inhibited T cells may be always present in mice, and the activation of these cells improved the immunity and survival rate of mice.
Conceptualization by Chuangfu Chen; methodology by Peng Wu; software by Peng Wu, Jiao Jiao; writing original draft preparation by Peng Wu, Jiao Jiao; writing and editing by Peng Wu, Jiao Jiao; funding acquisition by Chuangfu Chen. All authors read and approved the final manuscript.
This research was funded by CX Collaborative Innovation 2011 Collaborative Innovation Special Project (Grant no. 0101-KC- 0003), Humanization of 2019 novel coronavirus nanobodies (Grant no. ZZZC202084B) and The Molecular Mechanism of the Brucella virulence factors in Persistent infection (Grant no. U1803236).
Ethical review and approval were waived for this study, due to studies not involving humans or animals directly.
Contributions from the authors of all the literature covered in this article are appreciated.
The authors declare no conflict of interest.
Citation: Chen C, Wu P, Jiao J (2021) A Novel PD-L1 Nanobody Validates that Immune Cell Suppressed by PD-L1 May Always Exist in the Body. Immunotherapy (Los Angel).7:182.
Received: 01-Nov-2021 Accepted: 15-Nov-2021 Published: 22-Nov-2021
Copyright: © 2021 Chen C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.