
Immunotherapy: Open Access
Open Access
ISSN: 2471-9552

ISSN: 2471-9552
Research Article - (2020)Volume 6, Issue 3
Objective: To evaluate the effects of Protocadherin-PC (PCDH-PC) gene expression on Epithelial Mesenchymal Transition (EMT) in prostate cancer cells.
Methods: Western blot analysis, morphological analysis, and in vitro wound closure assay were used to assess the effects of inhibition of PCDH-PC expression on EMT in the androgen-independent prostate cancer cell lines DU- 145 and PC-3.
Results: Inhibition of PCDH-PC expression promoted Mesenchymal Epithelial Transition (MET) of DU-145 and PC-3 cells, which changed the cytomorphology to resemble that of the androgen-dependent prostate adenocarcinoma cells LNCaP, and slowed down the growth rate.
Conclusions: Inhibition of PCDH-PC expression reduced the invasiveness of androgen-independent prostate cancer cells.
Protocadherin-PC (PCDH-PC or PCDHY); Prostate cancer; Mesenchymal–epithelial transition (MET)
The LNCaP prostate cancer cell line is made up of androgen- dependent prostate adenocarcinoma cells. However, prostate cancer cells involved in the advanced prostate cancer are usually androgen-independent. LNCaP cells can undergo neuroendocrine differentiation and become androgen-independent prostate cancer cells in Charcoal-Stripped Fetal Bovine Serum (CS-FBS), which can remove androgen in vitro [1]. Our previous study showed that the Protocadherin-PC (PCDH-PC or PCDHY) gene induces neuroendocrine differentiation of LNCaP prostate cancer cell line, and short interfering RNA (siRNA) targeting PCDH-PC blocks the expression of PCDH-PC gene, thereby interrupting the neuroendocrine differentiation of androgen-independent prostate cancer cells [2]. The DU-145 and PC-3 are androgen-independent prostate cancer cell lines. The effects of the DU-145 and PC-3 cells transfected with siRNA targeting PCDH-PC have not been reported. We here evaluated the effect of PCDH-PC inhibition in prostate cancer cells and demonstrated the presence of Epithelial– Mesenchymal Transition (EMT) in the transfected prostate cancer cells.
To evaluate in a pilot-study the efficacy and tolerability of DL- PDT with a thermosetting 5% ALA gel applied using a specific penetration enhancer mask device.
Cell lines
Human prostate cancer cells, DU145, PC-3 were obtained from American Type Culture Collection (Manassas, VA).DU145 cells were maintained in RPMI 1640, PC-3 cells in F12K. All media were supplemented with 10% Fetal Bovine Serum (FBS) and penicillin/ streptomycin unless noted.
Transfection protocols
Transfections for protein extractions were done in 35 cm2 dishes with cells plated 12 hours prior. Aliquots of plasmid (6 ug) were mixed with Lipofect AMINE 2000 (Invitrogen Life Technologies) in antibiotic- and serum-free medium and were applied to the cultures.
Protocadherin-PC siRNA
Protocadherin-PC siRNA were purchased from Thermo Fisher Scientific Dharmacon,Inc. siRNAs were transfected at 100 nmol/L final concentrations using Lipofect AMINE 2000 in serum-free medium. Forty-eight hours after transfection, cells were harvested and extracted for protein.
Protein extraction and nuclear isolation procedures
Monolayer cultures were washed and scraped into cold PBS and were pelleted by low-speed centrifugation. Cell pellets were extracted in radio immunoprecipitation assay buffer. Aliquots of whole cell extracts were assayed for protein using the Bio-Rad DC protein assay (Bio-Rad, Inc., Hercules, CA).
Western blot
Aliquots of cell extracts containing equal amounts of protein were electrophoresed on 10% polyacrylamide gels and proteins in the gel were electro transferred to PVD filters. Antibodies Anti-β-Actin and Anti-Vimentin used in Western blot analyses were obtained from Sigma, Anti-E-Cadherin was obtained from BD Pharmingen. Antibody dilutions were done according to the manufacturer’s recommendations and primary antibody binding was detected using horseradish peroxidase-labeled secondary antibody. Chemiluminescent detection of secondary antibody was done using Luminol reagent (Santa Cruz Biotechnology) and exposing the filters to film (Kodak XAR5). Bands on film were compared with prestained molecular weight markers to ascertain the identity of the detected protein.
Cytomorphological analysis
A Nikon microscope and imaging system was used for cytomorphological analysis in this study.
In vitro wound closure assay
An in vitro wound closure assay (scratch assay) was performed to measure the scratch cell coverages at different time points.
Results of western blot analysis
As shown in Figure 1, vimentin protein was highly expressed, and E-cadherin protein was downregulated in the DU-145 and PC-3 cells transfected with siRNA targeting the PTCH-PC.
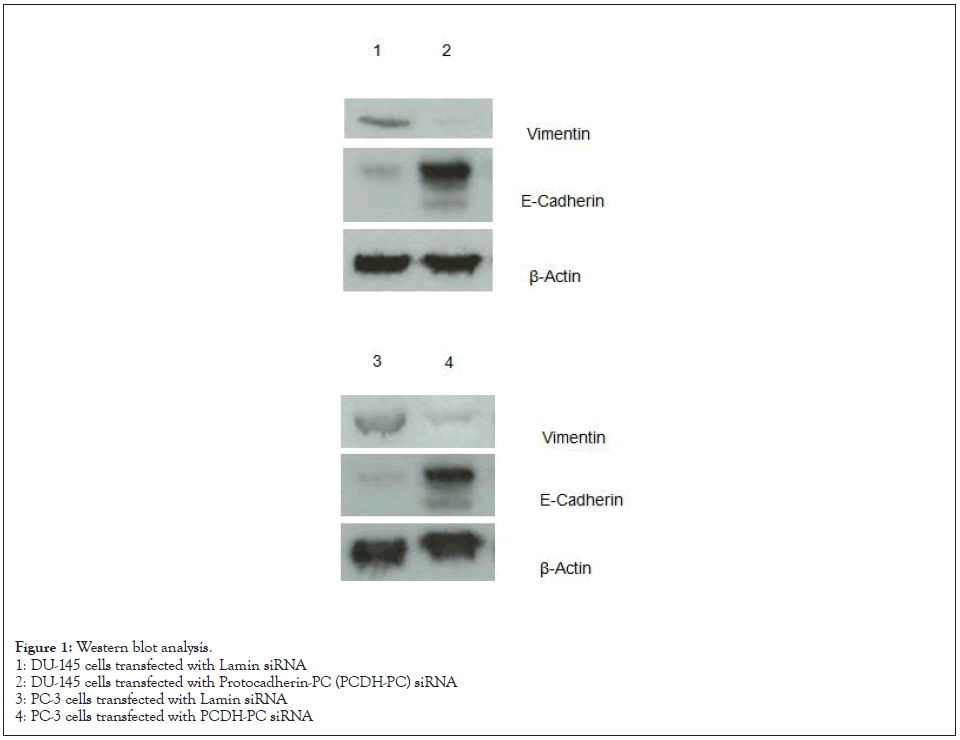
Figure 1: Western blot analysis.
1: DU-145 cells transfected with Lamin siRNA
2: DU-145 cells transfected with Protocadherin-PC (PCDH-PC) siRNA
3: PC-3 cells transfected with Lamin siRNA
4: PC-3 cells transfected with PCDH-PC siRNA
Cytomorphology
As shown in Figure 2, morphological changes were observed in the androgen-independent prostate cancer cell lines DU-145 and PC-3 that were transfected with siRNA targeting PTCH-PC, and the cells looked like androgen-dependent LNCaP cells.

Figure 2: Cytomorphological results.
1. DU-145 cells transfected with Lamin siRNA
2. DU-145 cells transfected with Protocadherin-PC (PCDH-PC) siRNA
3. PC-3 cells transfected with Lamin siRNA
4. PC-3 cells transfected with PCDH-PC siRNA
Results of in vitro wound closure assay
As shown in Figure 3, the recovery of cell scratches of DU-145 and PC-3 cells transfected with siRNA targeting PTCH-PC was significantly slower than untransfected cells.

Figure 3: In vitro wound closure assay.
1. DU-145 cells transfected with Lamin siRNA
2. DU-145 cells transfected with Protocadherin-PC (PCDH-PC) siRNA
3. PC-3 cells transfected with Lamin siRNA
4. PC-3 cells transfected with PCDH-PC siRNA
Prostate cancer is a common malignant tumor in men. The 5-year survival rate among patients diagnosed early can be as high as 90% [2]. However, 15%–20% of the patients may still have tumor invasion and metastasis. The 5-year survival rate of patients with metastatic prostate cancer is less than 30%. Therefore, it is important to clearly establish the mechanisms underlying invasion and metastasis of prostate cancer to improve the diagnosis and treatment and the prognosis of the patients. Tumor cells in carcinomas are more invasive and metastatic when epithelial tumor cells undergo EMT and take on mesenchymal rather than epithelial phenotypes [3].
EMT is the phenomenon by which epithelial cells transdifferentiate into mesenchymal cells under specific physiological and pathological conditions. This concept was first reported by Greenberg and Hay in 1982. They found that the lens epithelial cells developed pseudopodia in collagen gels and morphologically transformed into mesenchymal cells. EMT is considered a pathological process, leading to tumor progression and related to tumor invasion and metastasis. EMT is an important part of how tumors cells undergo acquire invasion and metastasis [4]. For this reason, EMT is considered a key step in metastasis of prostate cancer [5]. In the process of EMT, epithelial cells lose their polarity and their adhesion to surrounding cells and stromal cells weakens which in turn weakens cell-cell interaction and enhances cell migration and motor ability. In addition, cell phenotypes are altered as they lose the epithelial phenotypes, such as the expression of cytokeratin filaments and epithelial cell cadherin (E-cadherin) and acquire mesenchymal phenotypes, such as vimentin, fibronectin, and N-cadherin expression. Downregulated expression of E-cadherin results in poor cell adhesion, and upregulated expression of vimentin results in increased cell migration. The loss of E-cadherin expression and the increased vimentin expression have been found to be the most prominent features of EMT. In addition, EMT can be reversible, and this opposite process is called Mesenchymal– Epithelial Transition (MET) [6].
PCDH-PC is a gene specifically expressed in anti-apoptotic and androgen-independent prostate cancer cells. It discovered by Professor Ralph Buttyan in the early 2000’s, cloned in a laboratory at Columbia University Medical Center (New York City, NY, US) [7]. A later study by Yang et al. reported the important function of PCDH-PC, which induces neuroendocrine differentiation of androgen-dependent prostate cancer cells [1]. They also found siRNA targeting PCDH-PC could inhibit gene expression, thereby suppressing the neuroendocrine differentiation of prostate cancer cells. Neuroendocrine differentiation is prevalent in advanced prostate cancer [8], which is of great significance to the understanding human prostate cancer progression to the androgenresistant phase [9]. Few additional publications on PCDH-PC have been reported since its discovery. Apart from its relevance for hormonal resistance in prostate cancer, the effects of PCDH-PC in prostate cancer remain uncertain. A follow-up study by Yang et al. has shown that EMT is present in androgen-independent prostate cancer cells, which have cell morphology different from that of the androgen-dependent prostate cancer cell line LNCaP and have a faster growth rate. These findings were consistent with the results reported by Terry et al. [1,10,11].
Inhibition of PCDH-PC expression reversed EMT and led to MET, thereby suppressing the metastasis of prostate cancer. These study results opened up a new research area of PCDH-PC, which may be a suitable target gene for inhibition of prostate cancer invasion and metastasis and may be used as a diagnostic marker in prediction of the prognosis of prostate cancer.
Grant support: 2016 Anhui Province Human Society Hall Preferential Subsidy Program of Innovative Project for Overseas Students
Citation: Yang X, Jiang X, Ma X, Habuchi T, Guo Y (2020) A Novel Research Area of PCDH-PC in Inhibition of Prostate Cancer Cells Invasion and Metastasis through Epithelial: Mesenchymal Transition. Immunotherapy (Los Angel). 6:164.
Received: 29-Sep-2020 Accepted: 22-Oct-2020 Published: 29-Oct-2020 , DOI: 10.35248/2471-9552.20.6.164
Copyright: © 2020 Yang X, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.