
Journal of Clinical Trials
Open Access
ISSN: 2167-0870

ISSN: 2167-0870
Research Article - (2022)Volume 12, Issue 2
Objective: Assess the safety, tolerability and effects of the Peroxisome Proliferator-Activated Receptor δ (PPARδ) agonist REN001 on muscle recovery after limb immobilization.
Methods: Eligible healthy adults were randomly assigned 1:1 to REN001 100 mg twice daily or placebo for 28 days. Participants were leg-immobilized with a knee brace and used crutches from 1 to 14 days. Changes in muscle strength, gene expression from muscle biopsies and muscle Cross-Sectional Area (CSA) in the immobilized leg were evaluated. After 14 days of dosing the brace was removed and the subjects took study drug for another 14 days, gradually resuming regular physical activity. The primary pharmacodynamics endpoint was the change from baseline to day 21 in muscle strength as measured by knee extension.
Results: Twenty-four male participants were enrolled and treated, 12 in each treatment group. Four participants (16.7%, 2 in each group) prematurely discontinued. In the primary endpoint, REN001-treated subjects had greater mean increases from baseline to Day 21 in single knee extension strength vs. placebo (mixed models repeated measures and mixed model baseline covariate analysis P-values both <0.05). REN001-treated individuals had up to 3.5-fold increases from baseline from Day 14 in pyruvate dehydrogenase lipoamide kinase isozyme 4, angiopoietin-like 4, and solute carrier family 25 member 34, important PPARδ-regulated genes involved in mitochondrial function and biogenesis. No treatment group differences in the mean change from baseline in muscle CSA or muscle volume were observed. Adverse events were reported by 58.3% taking REN001 vs. 33.3% placebo; none were severe or serious and all resolved without sequelae.
Conclusion: REN001 was safe and well tolerated in this study. Data from this study in humans support the safety and purported action of PPARδ agonists by preventing muscle atrophy and increasing muscle strength, providing a rationale to evaluate REN001 in patients with mitochondrial myopathies.
REN001; PPAR delta agonists; Muscular atrophy; Randomized controlled trial; Phase 1 clinical trial; Healthy volunteers; Immobilization
PPARs are ligand-modulated transcription factors regulating gene expression of many cellular processes [1]. All 3 PPARs (α, γ and δ) are activated by lipids and have become targets in metabolic and cardiovascular pharmacotherapy [2]. PPARδ exerts control over genes involved in the synthesis, storage, mobilization and metabolism of fatty acids. In mouse muscles, genetic overexpression and pharmacological activation of PPARδ led to a shift in the number of fibers with high mitochondrial content and fatty acid oxidation improvement [3]. Increases in oxidative muscle fibers and improved running endurance were observed in untrained adult transgenic mice overexpressing a constitutively active PPARδ (VP16-PPARδ) in skeletal muscle [4]. In another report, mice treated for 4 weeks with a PPARδ agonist and exercise led to fatigue resistant oxidative muscle fibers and mitochondrial biogenesis, accompanied by improved physical performance [5]. Upon 8 weeks of PPARδ agonist treatment, a shift in energy substrate usage from glucose to fatty acid oxidation was apparent. These findings are consistent with increases in fatty acid metabolism observed with intense exercise training in mice [6].
Agents that increase mitochondrial enzyme activity or mitochondrial biosynthesis have been suggested as potential treatments for patients with mitochondrial myopathies [7]. REN001 (sodium (4-((E)-3-(4-fluorophenyl)-3-(4-(3-morpholin-4-yl-prop-1ynyl)phenyl) allyloxy)-2-methylphenoxy)acetate (Figure 1) was previously known as HPP593. REN001 is a PPARδ agonist currently being developed for the treatment of primary mitochondrial myopathies, glycogen storage disorder V, and fatty acid oxidation disorders. REN001 is highly selective for PPARδ and at the doses being explored has minimal or no activity on PPARα and PPARγ. Even at very high concentrations, REN001 acts as a full agonist of PPARδ and only a partial agonist for PPARα and PPARγ.
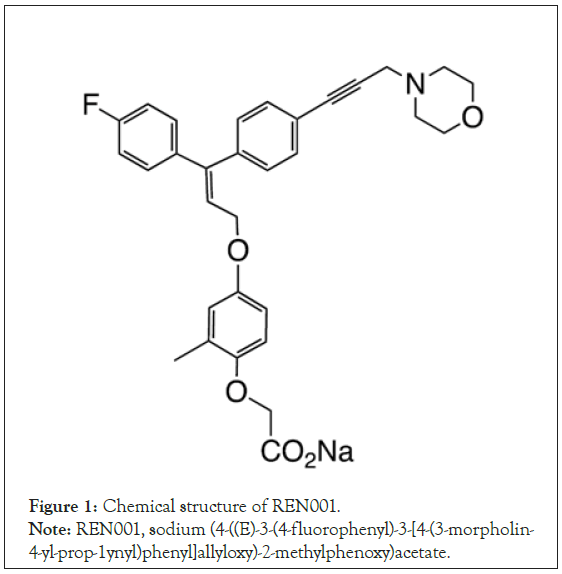
Figure 1: Chemical structure of REN001.
Note: REN001, sodium (4-((E)-3-(4-fluorophenyl)-3-[4-(3-morpholin-
4-yl-prop-1ynyl)phenyl]allyloxy)-2-methylphenoxy)acetate.
Findings from in vitro studies demonstrated that REN001 potently and selectively activated PPARδ in cell lines from humans, cynomolgus monkeys and rats increasing fatty acid oxidation. Nonclinical studies in mouse muscle showed that REN001 administration changed the expression patterns of PPARδ-regulated genes in pathways involved in the beta-oxidation of long chain fatty acids (carnitine palmitoyltransferase-1B) and mitochondrial biogenesis (PPAR gamma coactivator 1-α (PGC-1α)). In rat muscle, REN001 also increased the expression of angiopoietin-like 4.
The safety and pharmacokinetic characteristics of REN001 were evaluated in healthy adults and patients with obesity that had moderate dyslipidemia. Single doses of REN001 up to 250 mg and 14 days of 200 mg once daily and 100 mg twice daily (BID) were generally well tolerated (unpublished data). The current study was designed to determine the effect of REN001 on the recovery of muscle atrophy from leg immobilization. The primary objective was to determine the safety and tolerability of REN001 in healthy volunteers during and after limb immobilization. Secondary objectives were to investigate the effect of REN001 on muscle strength, muscle CSA, muscle volume, and biomarkers related to muscle function. An improvement in strength parameters was expected in REN001-treated individuals compared to placebo. Results from this proof-of-concept study are reported.
Standard protocol approvals, registrations and participant consents
The protocol, protocol amendments, informed consent forms and other documents requiring pre-approval were reviewed and approved by the Human Research Protection Office at Washington University School of Medicine and The University of Texas Medical Branch Institutional Review Board. Written informed consent was obtained from each individual prior to study enrollment. Study conduct adhered to Good Clinical Practice guidelines and all applicable regulatory requirements and ethical principles, including the Declaration of Helsinki. The study was monitored by a data safety monitoring board and was registered with ClinicalTrials.gov (NCT01524406).
Participants
This phase 1, randomized, parallel group, placebo-controlled study was conducted at 2 centers in the United States. Fifty participants were planned for enrollment, 25 per group; participants could be replaced upon sponsor approval.
Screening was conducted 28 days before study start; eligibility was reviewed again at prespecified times during the study. Ambulatory, non-smoking, healthy male volunteers 30 to 55 years of age with a Body Mass Index (BMI) from 18 to 30 kg/m2 were recruited for the study. Key exclusion criteria included abnormal laboratory values at screening, a recent history of body weight fluctuations, positive urine drug screens, drug dependency or illicit drug use, professional sport participation within 30 days of screening, donation of whole blood within 90 days of dosing, and donation of plasma within 30 days of dosing. Concomitant medications within 14 days of dosing were not allowed unless approved by the sponsor. Those who had enrolled in another clinical trial within 90 days or had previously received REN001 were not allowed to participate.
Randomization and masking
Participants were randomly assigned 1:1 to receive either REN001 100 mg BID or matching placebo for 28 days using a computergenerated randomization schedule provided by the sponsor. The principal investigator, study center staff, and participants were blinded to treatment; the protocol was amended so that sponsor personnel were not blinded. This change was made to allow investigation of observations concerning adherence to the protocol, discussed further in the pharmacodynamic results.
REN001 and placebo were provided as capsules identical in appearance, taken orally with 240 ml of water prior to meals. Study drug was administered at the study site on Days 1 (PM dose), 6 (AM dose), 13 (PM dose), and 14, 15, 16, 29 (AM doses). At all other times study drug was self-administered just before breakfast and dinner.
Dates and times were recorded in a diary, with compliance determined from the diaries and capsule counts. Study drug supplies were packaged according to the randomization codes by a contract research organization. Each dispensed bottle was labeled with the randomization number. Individual capsules were redispensed from these bottles by the study site staff, maintaining the blind.
Assessments
The total study duration for each subject was 42 days (Figure 2). Participants stayed overnight from Day 1 and Days 13 to 16. Standardized meals, beverages and snacks were provided during the domicile periods. On Day 1, participants were leg-immobilized using a knee brace with 30° of flexion on the left leg to allow driving. Walking crutches were provided to prevent weight bearing on the immobilized leg. An accelerometer was issued at screening and was used to count the number of steps per day. Participants were to walk 4000 to 6000 steps each day for the duration of the leg immobilization period, and recorded accelerometer readings each evening before bedtime. The brace was removed on Day 14, and participants resumed normal physical activity gradually from Days 16 to 29. After stopping study drug on Day 29, participants were to resume regular daily activity gradually for another 2 weeks.
Figure 2: Study schematic and disposition of participants.
Consumption of prescribed medications or over the counter products was prohibited from 14 days prior to dosing and throughout the study, with the exception of the treatment of an adverse event. Participants were not to consume alcohol or caffeinecontaining food and beverages 3 days before each study visit and domicile periods.
Participants were asked to fast for at least 8 hours prior to study visits on Days 1, 6, 14, 21, 29 and 42. The muscle strength test, physical performance test and punch biopsies from the quadriceps femoris (for biomarker assessment) occurred on Day 1 in the morning prior to dosing and on Days 14, 16, 21, 29, and 42. Participants underwent Magnetic Resonance Imaging (MRI) of the thigh to determine muscle CSA and muscle volume on Days 1, 14, 21, 29 and 42. The muscle strength test was the maximal amount of weight that the participant was able to lift for 1 repetition using a Hoist multi-station weight machine for a leg press, knee extension, knee flexion, and bench press. Isokinetic testing of knee extension and flexion was conducted (Cybex, Life Fitness, Rosemont, IL) to assess deficiencies in rapid strength recruitment. Isokinetic testing of the knee extensors and flexors was performed at 0 degrees, 60° and 180°. The highest 2 values from 4 to 5 repetitions were analyzed. A modified physical performance test was conducted to objectively evaluate physical performance.
MRIs were acquired on a 1.5-T superconducting Siemens MRI scanner (Siemens, Iselin, NJ) at the study site. Muscle volumes were determined by segmenting the cross-sectional muscle areas for each slice after correcting/subtracting intramuscular fat, using MATLAB (Mathworks, Natick, MA) and summing the area by slice thickness for all slices. Concomitant medications, clinical laboratory tests, vital signs, and 12-lead Electrocardiograms (ECGs) were evaluated at screening and on Day 1, 6, 14, 16, 21, 29 and 42. Laboratory tests included clinical chemistry, hematology, Creatine Kinase (CK), troponin I, C-Reactive Protein (CRP) and insulin. Serology was assessed at screening and urinalysis was conducted at screening and on Days 1 and 42. Samples for plasma trough concentrations were obtained on Days 6, 14, 15, 16, 21 and 29 and were analyzed by Pharsight Consulting Services using a validated bioanalysis method. Treatment-emergent adverse events were collected from Days 1 through 42. Body weight and BMI were measured at screening and on Days 1, 29, and 42, and physical examinations were performed regularly.
Statistics
The sample size of 50 participants was based on study precedent and feasibility rather than statistical considerations. Baseline was defined as the last assessment before the first dose of study drug; the maximum of the 2 latest measures prior to randomization was used for clinical measures. All statistical analyses were conducted using SAS version 9.3 (SAS Institute, Cary, NC). Demographic characteristics, safety assessments and pharmacokinetic parameters were summarized descriptively. Safety analyses were based on the safety analysis set, defined as all participants who took at least 1 dose of study drug. Adverse events were coded using the Medical Dictionary for Regulatory Activities (MedDRA) version 15.0. QT intervals were corrected for heart rate using Bazzet’s formula (QTcB) and Fridericia's formula and summarized categorically.
The primary pharmacodynamic endpoint was the change from baseline to Day 21 (1 week after removal of the brace) in muscle strength as measured by knee extension. Statistical analysis of the primary endpoint used PROC MIANALYZE with multipleimputation methods for coping with missing data (based on recursive regression). Supportive analysis used Mixed-Models Repeated Measures (MMRM) with a main-effects model including the baseline measure as a covariable and Days 14 and 21 as repeated measures. Analysis of Covariance (ANCOVA) was used with a main-effects model for single-value response measures. Interactions were examined and the impact on study conclusions assessed. The primary analysis of knee extension used main-effects models regardless of the significance of the interaction terms.
Parametric assumptions of normality of errors and homoscedasticity were examined. In the unlikely event that parametric assumptions were found to be unwarranted, rank analogues were to be advanced. Interaction terms were included in a supportive analysis. If interaction terms were significant, the impact on analysis conclusions was to be assessed. Regardless of the significance of interactions, the primary model for any variable was main effects. Supportive analysis was conducted using main-effects ANCOVA with baseline as a covariable at each assessment time. An analysis excluding participants with invalid data was done as a supportive analysis. Additionally, a supportive intent-to-treat analysis on the full dataset without exclusion occurred to ensure robustness of study conclusions against methods used to address invalid data. Invalid data were set to missing and imputed using multiple strategies. An analysis was also conducted that excluded participant values set to missing due to classification of invalid data.
The primary analysis on changes in muscle size using CSA over 28 days of treatment utilized MMRM to examine slopes over the post-treatment period. A main-effects model was used including baseline as a covariate, and including treatment. Interaction terms were examined; the primary model was to be main effects regardless of the significance of interaction terms. Statistical analysis on other measures utilized ANCOVA with baseline as a covariate at each assessment time. Simple models were also used including a 2-sample t-test at each time point.
Muscle volume was examined as a supportive endpoint for the muscle size measure. Muscle strength was also analyzed by change in isometric measures, using the maximum value obtained on the day of assessment. Changes from baseline in serial muscle biopsies obtained at Days 14, 16, 21, and 29 were analyzed for PGC-1α downstream gene profile, microRNA analysis, gene expression analysis, protein content, enzyme analysis, and muscle fiber size.
Data availability
The datasets from this study are not publicly available but are available from the corresponding author upon reasonable request.
Study population and exposure
The study was conducted between March 19, 2012 and March 22, 2013. Although it was planned to enroll subjects at 2 sites, 1 site (Washington University, St. Louis Missouri) was able to enroll the majority of participants into the study. The sponsor stopped the study after 24 of 50 planned participants had been enrolled due to slow accrual. Participant baseline characteristics were similar across treatment groups (Table 1). All 24 healthy volunteers enrolled in the study (100%) took at least 1 dose of study drug, with 10 participants in each group (83.3%) completing the full 28 days of dosing (Figure 2).
| Demographic characteristic | Placebo (n=12) | REN001 (n=12) |
|---|---|---|
| Age, years | ||
| Mean (SD) | 39 (8.2) | 42 (8.9) |
| Median (range)a | 38 (30, 56) | 43 (30, 53) |
| Sex, number (%) | ||
| Male | 12 (100) | 12 (100) |
| Race, number (%) | ||
| Black or African American | 6 (50.0) | 7 (58.3) |
| White | 6 (50.0) | 5 (41.7) |
| Ethnicity, number (%) | ||
| Non-Hispanic or Latino | 12 (100) | 12 (100) |
| Height, cm | ||
| Mean (SD) | 179.7 (7.16) | 179.2 (8.46) |
| Median (range) | 179 (170, 193) | 180 (164, 197) |
| Weight, kg | ||
| Mean (SD) | 82.8 (9.99) | 78.1 (8.42) |
| Median (range) | 83.6 (67.2, 97.6) | 76 .1 (68.3, 95.6) |
| BMI, kg/m2 | ||
| Mean (SD) | 25.6 (2.69) | 24.4 (2.83) |
| Median (range) | 25.9 (20.5, 29.3) | 24.7 (21.0, 29.1) |
Note: SD: Standard Deviation, Data are summarized based on the safety analysis set; aOne 56-year-old placebo-treated participant who reported his age as 55 years at screening was allowed to continue in the study
Table 1: Demographic characteristics at baseline.
Four participants (16.7%) prematurely discontinued from the study, 2 in each group. After the study began it was discovered that 2 men in the placebo group (16.7%) should have failed screening; both withdrew after 21 days of participation. In the REN001 group 1 participant (8.3%) was discontinued from the study due to an adverse event of elevated CK and another participant (8.3%) was withdrawn due to noncompliance.
The mean (SD) total dose of REN001 administered was 4883 (1590.2) mg, with 23 participants (95.8%) missing at least 1 dose of study drug. The mean number ± SD of doses missed was similar in the 2 groups, 5 ± 2.6 for placebo and 4 ± 1.5 for REN001. Inter-participant coefficient of variation values for plasma trough concentrations were moderately high (Table 2).
| Study day | |||||||
|---|---|---|---|---|---|---|---|
| 6 | 14 | 15 | 16 | 21 | 29 | 42 | |
| Number of participants | 11 | 11 | 10 | 10 | 7 | 9 | 10 |
| Mean | 4878 | 4101 | 3502 | 3378 | 4579 | 3977 | 14 |
| Standard deviation | 2697 | 2211 | 1209 | 2040 | 2006 | 1354 | 9 |
| CV (%) | 55.3 | 53.9 | 34.5 | 60.4 | 43.8 | 34.1 | 64.5 |
| Median | 4270 | 3939 | 3906 | 3236 | 4221 | 4501 | 12 |
| Minimum | 248 | 8 | 1311 | 745 | 2049 | 1424 | 0 |
| Maximum | 10299 | 8221 | 4725 | 7219 | 7278 | 5242 | 34 |
| Geometric mean | 3768 | 2379 | 3266 | 2745 | 4198 | 3683 | 14 |
| CV geometric mean (%) | 127 | 616.7 | 44.4 | 84.4 | 48.4 | 48.5 | 46.1 |
Note: CV: Coefficient of Variation, Data is summarized for the pharmacokinetic analysis set, defined as all participants with a sufficient number of assay results above the limit of quantitation.
Table 2: REN001 plasma trough concentrations (ng/ml) over time.
Safety
Eleven participants overall (45.8%) reported 29 treatment-emergent adverse events during the study, 7 (58.3%) in the REN001 group and 4 (33.3%) in the placebo group (Table 3); all adverse events (100%) resolved without complications. Headache, rash, and postprocedural hematoma were the most frequently occurring adverse events, each reported for 2 REN001-treated individuals (16.7%). There were no serious or severe adverse events. The participant with elevated CK that led to study discontinuation had a baseline value of 257 IU/l increasing to 1479 IU/l on Day 5 (normal range 26 to 308 IU/l). The event resolved 8 days after onset. The increase may have been caused by strenuous exercise but was considered possibly related to study drug. Additional analyses of CK, troponin I and CRP values from both groups did not indicate a REN001 treatment effect.
| Placebo | REN001 | |
|---|---|---|
| n = 12 | n = 12 | |
| Number of adverse events | 8 | 21 |
| Number of participants (%) with at least 1 adverse event | 4 (33.3) | 7 (58.3) |
| Severe adverse event | 0 | 0 |
| Moderate adverse event | 1 (8.3)a | 2 (16.7)b |
| Mild adverse event | 3 (25.0) | 5 (41.7) |
| Serious adverse event | 0 | 0 |
| Death | 0 | 0 |
| Discontinued due to an adverse event | 0 | 1 (8.3)c |
| Considered possibly or probably drug related | 3 (25.0) | 5 (41.7) |
| Adverse events reported by 2 or more participants taking REN001 (MedDRA preferred term) | ||
| Headache | 1 (8.3) | 2 (16.7) |
| Post-procedural hematoma | 0 | 2 (16.7) |
| Rash | 1 (8.3) | 2 (16.7) |
Note: Data were summarized using the safety analysis set. Adverse events were coded using the Medical Dictionary for Regulatory Activities (MedDRA), version 15.0; aFood poisoning, unlikely related to study drug; bAbdominal pain and constipation in 1 participant, both unlikely related to study drug, and elevated creatine kinase in another participant, possibly related to study drug; cElevated creatine kinase, possibly related to study drug.
Table 3: Summary of treatment-emergent adverse events.
Mean low-density lipoprotein cholesterol, total cholesterol and triglyceride values decreased in both groups during the study, with larger decreases observed in REN001-treated participants. No other changes in mean clinical laboratory values were observed. Similar proportions of participants from each group had transient laboratory values outside normal range. No changes in vital signs or ECGs were reported as adverse events, and no clinically important changes were observed. Mean pulse rates in the REN001 group increased from baseline, ranging from 3.5 to 16.27 beats per minute. One REN001-treated participant (8.3%) had a QTcB value>450 msec and 1 placebo-treated participant (8.3%) had a QTcB increase >30 msec during the study. There were no QTcB values greater than 480 msec or a change in QTcB greater than 60 msec.
Pharmacodynamics
As 1 site enrolled only 2 participants and used a different methodology, the pharmacodynamic analysis excluded participants from that site. In addition, an unblinded review by the sponsor indicated that some placebo-treated participants had substantial increases in muscle volume under the protocol-required 14-day limb immobilization. This finding was inconsistent with the expectation that limb immobilization would lead to muscle atrophy. It was determined that participants probably removed their leg braces for showers or other activities, invalidating the data. A blinded data review was conducted to identify those with substantial increases in muscle volume during limb immobilization, yielding an SD of 8.26% for the available data for percent change from Days 1 to 14 at a treatment-blind snapshot. Data from Days 14, 16 and 21 were therefore considered missing for individuals who had an increase in muscle volume greater than 8.3%, regardless of their assigned treatment group.
Day 16 was planned as an assessment of biomarkers. Clinical assessments on this day were considered uninterpretable because the protocol limited exercise for biomarker optimization and there was likely residual muscle soreness from the Day 14 biopsy. Therefore Day 16 clinical data were not planned for summary statistics and were not used in the last observation carried forward (LOCF) analysis. In the primary endpoint analysis, participants receiving REN001 had a greater mean increase from baseline to Day 21 in knee extension strength compared to placebo (MMRM and mixed model baseline covariate analysis p-values both <0.05, Figure 3, Table 4). In addition, participants in the placebo group had lost more knee strength during the immobilization period compared to the REN001 group (p=0.01). At Day 29, the change from baseline in mean knee extension strength was numerically higher for the REN001 group.
| Baseline | Day 14 | Day 21 | Day 29 | Day 42 | |
|---|---|---|---|---|---|
| Number of participants | |||||
| REN001 | 11 | 10 | 8 | 9 | 9 |
| Placebo | 11 | 11 | 11 | 10 | 10 |
| Change from baseline, mean (SD) | |||||
| REN001 | −5.86 (28.818) | 32.82 (28.158) | 25.58 (40.598) | 37.96 (37.442) | |
| Placebo | −36.20 (34.339) | 2.72 (23.826) | 13.73 (25.712) | 30.00 (42.913) | |
Table 4: Change from baseline in muscle strength over time.
Figure 3: Change in muscle strength over time.
Note: Results based on recursive regression imputation in the full analysis set; a: p-value derived from a mixed model with baseline value as covariate and including Days 14 and 21 as repeated measures and b: p-value derived from a mixed model with baseline value as covariate; 
Similar results were obtained when the muscle strength primary endpoint was analyzed using the per-protocol LOCF imputation. At Day 21, the mean ± SD change from baseline for REN001-treated participants was 28.61 ± 33.333 lb compared to 3.44 ± 28.440 lb in the placebo group (MMRM and mixed model baseline covariate analysis P-values both ≤.05). The placebo group lost more knee strength during limb immobilization (-34.38 ± 40.703 lb at Day 14) compared with REN001 (-5.28 ± 30.502 lb; p=0.04). Participants in the REN001 group had numerically greater mean changes in mean knee strength at Day 29 (28.06 ± 41.416 lb) compared to placebo (18.13 ± 31.075 lb). No treatment group differences were observed in the mean change from baseline in muscle CSA, muscle volume, or isometric knee extensor (Table 5).
| Assessment | Baseline mean (SD) | Change from baseline, mean (SD) | ||||||
|---|---|---|---|---|---|---|---|---|
| Day 14 | Day 21 | Day 29 | ||||||
| Placebo (n=11) | REN001 (n=11) | Placebo (n=11) | REN001 (n=10) | Placebo (n=10) | REN001 (n=8) | Placebo (n=8) | REN001 (n=9) | |
| MRI muscle CSA (mm2) | 12.4 (2.24) | 12.2 (2.40) | −0.3 (1.37) | −0.20 (0.86) | −0.6 (1.12) | −1.1 (0.67) | −0.98 (1.72) | −1.01 (1.19) |
| MRI muscle volume (ml) | 899.7 (155.82) | 873.5 (167.30) | −22.1 (88.67) | −17.9 (58.90) | −45.20 (81.18) | −71.88 (48.87) | −72.5 (125.72) | −68.0 (84.11) |
| Isometric knee extensor peak torque | ||||||||
| 0° | 143.7 (43.30) | 130.8 (33.13) | −21.2 (28.60) | −12.1 (27.22) | 1.6 (33.55) | −5.3 (19.73) | 0.9 (19.76) | −3.3 (17.50) |
| 60° | 123.3 (32.85) | 118.6 (31.06) | −28.3 (34.75) | −24.8 (31.22) | −11.3 (28.10) | −16.2 (20.77) | −11.3 (16.89) | −9.8 (15.07) |
| 180° | 98.6 (21.92) | 81.4 (18.34) | −21.9 (29.41) | −15.5 (21.35) | −9.5 (19.99) | −9.1 (13.29) | −6.2 (10.48) | −2.5 (13.66) |
Note: CSA: Cross-Sectional Area; MRI: Magnetic Resonance Imaging; SD: Standard Deviation; Data summarized are based on the full analysis set.
Table 5: Other pharmacodynamic endpoint results.
Biomarkers
When muscle biopsies were analyzed for changes in mRNA expression of PPARδ-regulated genes involved in mitochondrial biogenesis and function, samples from REN001-treated individuals had up to 3.5-fold increases from baseline from Day 14 in pyruvate dehydrogenase lipoamide kinase isozyme 4 (PDK4), angiopoietinlike 4 (ANGPLT4), and solute carrier family 25 member 34 (Figure 4). As described above in the Day 16 assessments, biomarker analysis was considered unfruitful.
Figure 4: Expression of peroxisome proliferator-activated receptor delta-regulated genes from muscle biopsies.
Note: Changes from baseline in serial muscle biopsies, gene expression analysis (Global Gene Array); 
In the present study, treatment with the PPARδ agonist REN001 was safe and well tolerated. The frequency of treatment-emergent adverse events was similar in the 2 treatment groups and no clinically significant effects were seen in laboratory, ECG or physical examinations. The effect of REN001 on lipid values was not unexpected and is consistent with other findings.
REN001 preserved skeletal muscle strength in healthy volunteers undergoing 1-legged knee immobilization for 14 days. Compared with placebo, serial muscle biopsies obtained from the REN001- treated individuals showed clinically meaningful increases in the mRNA expression of the genes for from Day 14 onwards for PDK4 and ANGPTL4, important PPARδ-regulated genes involved in mitochondrial function and biogenesis [8,9].
It is well established that during short periods of bed rest or limb immobilization, healthy individuals experience rapid loss of skeletal muscle mass leading to decreased muscle strength [10,11] and reduced functional capacity [12-15]. The development of sarcopenia in older adults is thought to be partially attributed to muscle loss from short periods of immobility due to illness or injury [16]. In the primary endpoint, REN001-treated participants had a significantly greater mean increase from baseline to Day 21 in knee extension strength compared to placebo (Figure 3). In contrast, participants in the placebo group lost knee strength during the immobilization period compared to baseline and to the REN001 group. Two weeks after brace removal (Day 29), the change from baseline in mean knee extension strength was relatively similar for both treatment groups. It should be noted that study participants were allowed to resume physical activity after brace removal. As physical activity modulates mitochondrial content in healthy individuals [17] resuming physical activities possibly led to improvements in mitochondrial capacity and muscle strength.
No treatment group differences were observed in the mean change from baseline in muscle CSA or muscle volume. This finding is inconsistent with reports by others [18] and suggests that the changes in muscle strength observed with immobilization might have been related to functional rather than gross anatomical changes. Although magnetic resonance T2- or T1-weighted imaging is routinely used to evaluate muscular injury or edema occurring at a macroscopic scale, regular MRI methods used in the clinical setting is unreliable for detecting changes at a cellular level [19]. Pathologies such as weakness and muscular fatigue are typically characterized by disruptions that cannot be reliably assessed with regular MRI methods. Recently developed capabilities of microstructural imaging may enable probing of changes at the cellular level [20].
PPARδ controls genes involved in cellular metabolic processes such as glucose homeostasis, fatty acid synthesis and storage, and fatty acid mobilization and metabolism. PPARδ is the most abundant PPAR isoform in skeletal muscle and has a higher expression in oxidative type I muscle fibers compared with glycolytic type II muscle fibers [21]. Skeletal muscle atrophy after leg immobilization is associated with mitochondrial dysfunction. In experimental mice models of muscle atrophy, hindlimb immobilization for 2 weeks caused reductions in mitochondrial density [22]. Both genetic overexpression and pharmacological activation of PPARδ in mouse muscles resulted in increases in the number of fibers with high mitochondrial content and improved fatty acid oxidation [3]. Overexpression of a constitutively active PPARδ in skeletal muscles of transgenic mice pre-programed an increase in oxidative muscle fibers, enhancing running endurance in untrained adult mice [4]. The PPARδ agonist GW501516 combined with 4 weeks of exercise synergistically induced fatigue-resistant oxidative muscle fibers and mitochondrial biogenesis in mice and enhanced physical performance [5]. Consistent with these findings, in vitro studies using cell lines from human and rat muscle showed that REN001 potently and selectively activated PPARδ and increased fatty acid oxidation. We postulate that REN001 may prevent the muscular impairment associated with immobilization by increasing fatty acid oxidation and promoting mitochondrial biogenesis.
PPARδ heterodimerizes with the retinoid X receptor and binds to PPAR response elements to modulate the expression of multiple genes critical in fatty acid oxidation, glucose metabolism, and inflammation [23,24]. REN001-treated participant muscle biopsies had greater mRNA expression of PDK4 and ANGPTL4 than those from participants receiving placebo. Interestingly, PDK4 is highly expressed in skeletal muscle [25,26] and combined activation of AMP-activated protein kinase (AMPK) and PPARδ caused increased PDK4 expression in mouse muscle that was associated with improved fatty acid oxidation during exercise [27]. Moreover, ANGPTL4-deficient mice show low exercise endurance and ANGPTL4 has been shown to stimulate AMPK signaling that increases mitochondrial oxidative capacity in skeletal muscle cells [28].
One limitation of the study is the small number of subjects at a single academic center. While the protocol planned to enroll 50 subjects at 2 sites, the requirements for limb immobilization and multiple muscle biopsies made enrollment difficult. Muscle atrophy caused by immobilization is likely the result of both an increase in myofibrillar protein breakdown and a suppression of myofibrillar protein synthesis [29]. Although mRNA microarray analysis has proven to be a powerful method for the analysis of gene expression patterns in human tissues, we did not specifically analyze the content of myofibrillar proteins in the muscle biopsies. Similarly, the limiting factor used in the current study included the immobilization of only 1 leg while the rest of the body remained mobile.
As such, we cannot account for potential effects of other factors involved in muscle mass homeostasis, such as insulin sensitivity. Nevertheless, significant positive effects in preserving skeletal muscle strength following immobilization were observed with REN001 treatment. Our findings provide further support that PPARδ-mediated stimulation of mitochondrial activity and biogenesis with REN001 can prevent muscle strength loss and that REN001 warrants further evaluation in patients with rare monogenic nuclear or mitochondrial DNA disorders and polygenic diseases associated with diminished mitochondrial function.
The authors were assisted in the preparation of this manuscript by Muriel Cunningham, professional medical writer compensated by Reneo. Ms. Cunningham wrote an initial draft and manuscript revisions based on input from the authors, and edited the manuscript per the journal requirements.
This work was funded by High Point Pharmaceuticals, LLC (the original study sponsor). This publication was prepared by Reneo Pharmaceuticals Inc., the current owner of REN001.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Davies M, Dorenbaum A, Wang S, Mittendorfer B (2022) A Phase 1 Randomized, Placebo-Controlled, Parallel Study of the Novel Peroxisome Proliferator-Activated Receptor δ Agonist REN001 in Healthy Volunteers during and After Limb Immobilization. J Clin Trials. 12:495.
Received: 14-Mar-2022, Manuscript No. JCTR-22-16254; Editor assigned: 16-Mar-2022, Pre QC No. JCTR-22-16254 (PQ); Reviewed: 30-Mar-2022, QC No. JCTR-22-16254; Revised: 05-Apr-2022, Manuscript No. JCTR-22-16254 (R); Published: 12-Apr-2022 , DOI: 10.35248/2167-0870.22.12.495
Copyright: © 2022 Davies M, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.