Journal of Infectious Diseases & Preventive Medicine
Open Access
ISSN: 2329-8731
ISSN: 2329-8731
Research Article - (2022)Volume 10, Issue 5
Background: Cutanious Adverse drug reaction is any noxious change in skin which is suspected to be due to drug, occurs at doses normally used in humans for prophylaxis, diagnosis, therapy of disease or for modification of physiological function. Adverse drug reactions cause both morbidity and mortality.
Objectives: To study the clinical spectrum of cutaneous adverse drug reactions.
Methods: The study was carried out in the Department of Dermatology of Jaynagar general hospital, banglore from June 2016-July 2019. All the patients attending the Dermatology Outpatient Department and the patients admitted in the wards with suspected cutaneous adverse drug reactions to systemic drugs were included in the study. A detailed clinical history, including the history of drug intake was noted. Each case was assessed for its causality by using the WHO definitions.
Results: Patients with drug reactions were found to be more commonly female (66.28%) than male (33.72%). Antiretroviral drugs were found to be the most frequent cause of adverse cutaneous drug reactions (22.35%). Followed by non-steroidal anti-inflammatory drugs (16.86%). Acute urticaria (53.33%) was the most common clinical condition among all patients CADR followed by FDE (19.21%). most cases are seen after 24 hours to 1 week is 26.27%.
Conclusion: Adverse cutaneous drug reactions in our study population were mainly induced by anti-retroviral drugs and non-steroidal anti-inflammatory drugs. The most common forms of cutaneous adverse drug reactions were found to be acute urticaria, fixed drug eruptions, and maculopapular rashes.
Cutanious adverse drug reactions, Fixed drug eruption, Stevens-Johnson syndrome
Adverse Drug Reactions (ADRs) are undesirable and typically unanticipated reactions independent of the intended therapeutic purpose of a medication, that may result in significant morbidity and even mortality [1]. ADR occurring in 2%-3% of inpatient and in approximately 2% of outpatient patients referred for dermatologic evaluation; approximately 2% of ADRs are considered severe or fatal cutaneous drug eruptions are the most common type of adverse reactions to drug therapy, with an incidence rate of 2%-6% [2]. The 75% to 95% of cutaneous drug eruption are maculopapular rash/drug rash followed by utricaria. Non-Steroidal Anti-Inflammatory Drugs (NSAIDs), antibiotics and antiepileptics, have drug eruption rates approaching 1%-5% [3]. Drug reactions are more common in women, and increase with age and the number of medications used [4]. Age (children and elderly), gender, multiple medications, multiple comorbid conditions, inappropriate medications that were prescribed and used, improper monitoring, end-organ dysfunction, altered physiology, prior history of ADR’s, extent (dose) and duration of exposure, genetic predisposition are the various risk factors [5]. The clinical presentation of drug related cutaneous eruption ranges from mild rash to severe rash besides causing life-threatening reactions. Serious reactions include angio-oedema, erythroderma, Stevens-Johnson syndrome and toxic epidermal necrolysis [6]. Sometimes drug eruptions occur as part of a multiorgan involvement like drug-induced systemic lupus erythematosus, acute generalized exanthematous pustulosis,serum sickenss. Whereas maculopapular rashes and urticaria are among the most common cutaneous drug reactions, anaphylaxis, Stevens-Johnson syndrome, and toxic epidermal necrolysis may result in mortality [7]. The drug induced skin reactions also increases the health cost and compromise the quality of life [8]. The purpose of this study was to examine the clinical characteristics and purported etiologic agents for ADRs in our patient population [9].
A descriptive, prospective case-series study was performed during June 2016-July 2019; two hundred and thirty five consecutive inpatient and outpatient subjects with a diagnosis of ADR referred to the dermatology department in Jaynagar general hospital. All patients suspected of having an ADR were clinically evaluated by an attending dermatologist. Each patient was informed of the nature of the study and signed a consent form approved by institutional ethics committee. Confidentiality and anonymity of the patient’s information were maintained during and after the study. The study was performed in accordance with the ethical guidelines of the 1975 Declaration of Helsinki. Inclusion criteria:
• Patients of all ages, belonging to both genders presenting to dermatology services with skin reactions following intake of any drug.
• Patients referred from other clinical specialties to Dermatology department for the treatment of drug induced reactions.
Exclusion criteria are patient who are not willing to participate in study. Patient with any other skin conditions
During the study 255 patient are included in the study. Among them 86 (33.72%) are male and 169 (66.28%) are female. the mean age of the patient was 42. The diagnosis of cutanious adr purely on clinical manifestation was 204 (80%) and histological conformation was 51 (20%) [3]. ADR was most frequently seen in patient with age group 30-50 years of age. About 43% of skin manifestation is seen in this age group.
Females (66.27%) are more common for drug induced skin reactions than male (33.72%). onset of reaction (Cutanious) is most commonly seen after 24 hour before 1 week is 37.25% followed by <24 hour was 26.27%. 15 (5.88%) patients have previous allergy recorded (Figure 1).
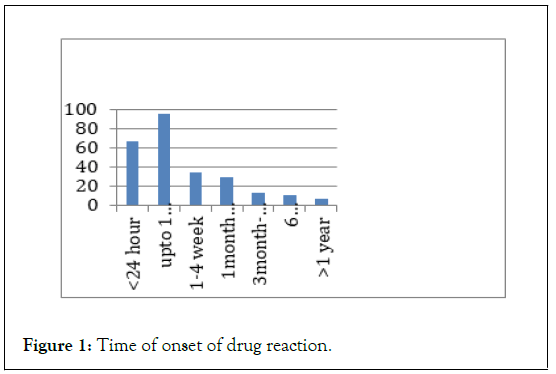
Figure 1: Time of onset of drug reaction.
Clinical manifestation of cutanious ADR
Clinical manifestations of cutaneous adverse drug reactions Among all patients 136 patient (53.33%) develop urticaria eruptions about 60% due to antibiotics, 35% due to NSAIDs (Table 1). among these antiretroviral drugs and NSAID are the leading causes of cutanious ADR. Abacavir is the most common drug among antiretroviral therapy followed by ibuprofen and paracetamol followed by diclofenac were the most common offending agents in NSAIDs respectively. In patients with fixed drug eruptions, 15(30.61%) were attributed to taking trimethoprim-sulfamethoxazole 9 (18.36%) to taking NSAIDs (Figure 2). Among the six patients with erythema multiform, two had history of taking trimethoprim-sulfamethoxazole. In this study, only one patient had a diagnosis of DRESS syndrome, which was attributed to phenytoin. In the study acute urticaria, Fixed drug eruption Exanthemata’s eruption was the most common cutanious manifestations (Table 2).
| Clinical feature | No of patient/ percentage | No of patient histologically confirmed |
|---|---|---|
| Acute urticaria | 136 | 4 |
| Fixed drug eruption | 49 | 12 |
| Exanthematous eruption | 37 | 9 |
| Erythema multiforme | 6 | 6 |
| Acute generalized exanthematous pustulosis | 5 | 5 |
| Vasculitis | 5 | 5 |
| Angioedema | 5 | |
| Erythroderma | 4 | 4 |
| Stevens-Johnson syndrome | 2 | 2 |
| Serum sickness | 2 | |
| Exfoliative dermatitis | 2 | 2 |
| DRESS syndrome | 1 | 1 |
| Photosensitive dermatitis | 1 | 1 |
| Total | 255 | 51 |
Table 1: Cutanious reaction seen in number of patient.
| Suspected drugs | Frequency |
|---|---|
| Antiretroviral drugs | 52 |
| NSAIDs | 43 |
| Antitubercular drugs | 32 |
| Antibacterial agents | 30 |
| Antiepileptics | 23 |
| Antileprotic drug | 11 |
| Antimalarial drugs | 10 |
| Antifungal | 7 |
| Hypolipaedemic drugs | 5 |
| Muscle relaxant | 5 |
| Antihypertensive | 5 |
| Antiulcer agents | 4 |
| OHA | 3 |
| Antimetabolite | 5 |
| Vaccine | 2 |
| Unknown | 8 |
| Fixed drug combination | 10 |
| Total | 255 |
Table 2: Incidence of drug in research population.
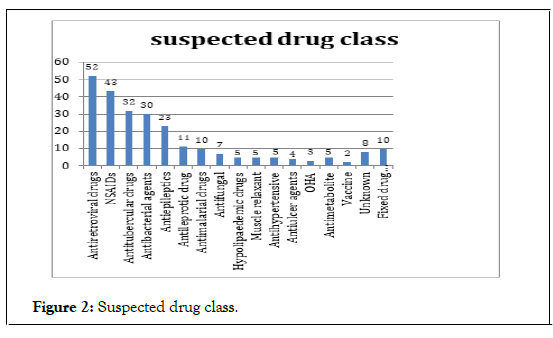
Figure 2: Suspected drug class.
Outcome of cutaneous ADRs
Out of 255 cutaneous ADRs, 58(22.74) cutaneous ADRs were continuing at the time of reporting. While 93 (36.47%) were recovering and 103(40.39%) had recovered and 1(0.39%) had deceased.
Causality assessment, severity, and preventability
Majority of cutaneous ADRs were categorized as possible (54.61%) followed by probable (45.38%) as per the Naranjo's algorithm. While WHO-UMC causality assessment showed maximum possible relationship (65.56%) with drugs followed by probable (32.99%). Majority of the cutaneous ADRs were moderately severe (167, 65.49%) while 77(30.19%) were mild and 11(4.3%) were severe in nature.
Cutanious ADR is one of the most common cause of ADR. The mean age of our patient was 42.3 comparable to the finding of French [10] and Italian studies [11] but older than Malaysian studies [12]. Similar with the previous studies done by Kacalak- Rzepka A et a l., to our stud y, w e foun d that wome ns a re more prone to cutanious ADR than in men [13].
In the present study majority (43%) of patient was in age group 30-50 years of age . majority of age group is in 3rd and 4th decades consistent with the findings of Sushma et al., in their 2005 study . A wide clinical spectrum of cutaneous adverse drug reactions was observed in our study [14]. Altogether we observed [13] different types of cutaneous adverse drug reactions [7].acute urticaria was the most common while Fixed drug eruption was the second most common drug eruption seen in our study similar to the finding of [15] Similar to current study [16] in 2007, the most common cutaneous clinical manifestations were maculopapular eruptions followed by fixed drug eruptions, and antibiotics and NSAIDs were the most commonly purported agents.
Consistant with most commonly seen after 24 hour before 1 week is 37.25% followed by <24 hour was 26.27% similar study was conducted.
Antimicrobials were the commonest drug category of drug involved in CADR observed by several authors. Collectively antimicrobial was the most common cause of CADR in our study.This is similar to the finding of other authors [14]. Antiretroviral agents like abacavir, antibiotics like beta lactams, quinolones ,sulphonamides are the major drugs causes ADR. Cotrimoxazole was the most common cause of drug eruptions in other studies [17-21]. The study reported that drug eruptions induced by trimethoprim-sulfamethoxazole were most commonly present on the genital area, while those induced by NSAIDs were most commonly seen on the lip. The Antibiotics such as peinicillin, cotrimoxazole, fluroquinolones drugs were responsible for the majority of drugs reactions according to study. in around 24% of cases. In our study, antibiotics were associated with both non serious as well as serious CADR like SJS. We observed that SJS was the most common type of serious CADR, similar to the observation of other authors [17,22]. DRESS syndrome is one of the severe forms of CADR. The drugs incriminated in the DRESS syndrome are anticonvulsants, allopurinol, sulphonamides and antibiotics [23]. We had observed 1 cases of dress syndrome, identified with help of histopathology which is caused by leflunomide. DRESS syndrome caused by Leflunomide was also reported by [24,25] In our study, six patients (5.88%) had previous CADR with the drug of similar category, four had recurrent FDE, one had exanthematous drug reaction and one patient got TEN for second time with ursodeoxycholic acid. It should be noted that our study does not address the mechanism of ADRs; In addition, a drug reaction cannot be confirmed without further testing, such as rechallenge, which was not performed in our study. Moreover, histologic examination may not reveal changes specific to a drug eruption, although biopsies may be helpful in distinguishing particular subtypes of reactions.
According to our results, adverse cutaneous drug reactions were mainly induced by beta-lactam antibiotics and NSAIDs. The most common forms of cutaneous ADRs in order of frequency were acute urticaria, fixed drug eruptions, and maculopapular rashes. In our study, the most common form of cutaneous ADR was found to be urticaria. Women’s were most susceptible to CADR while most cases are seen within 1 week of the study.
The authors declare that they have no competing interests.
Citation: Niraula R, Thoutoum PC, Devkota A, Poudel S (2022) A Prospective Study of Drug Induced-Skin Reactions: Patient Approaching Department of Dermatology in Secondary Care Hospital. J Infect Dis Preve Med. 10: 271.
Received: 24-Aug-2022, Manuscript No. JADPR-22-17071; Editor assigned: 26-Aug-2022, Pre QC No. JADPR-22-17071 (PQ); Reviewed: 09-Sep-2022, QC No. JADPR-22-17071; Revised: 19-Sep-2022, Manuscript No. JADPR-22-17071 (R); Published: 26-Sep-2022 , DOI: 10.35841/2329-8731.22.10.271
Copyright: © 2022 Niraula R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.