
Journal of Clinical Trials
Open Access
ISSN: 2167-0870

ISSN: 2167-0870
Research Article - (2020)
COVID-19 outbreak has put forward a huge challenge in front of researchers across the globe. Non availability of any vaccine or drug has worsened the situation and it has become a worst pandemic of modern times. Keeping scientific rationale and results of in silico studies, Researchers at Shreepad Shree Vallabh SSV Phytopharmaceuticals (SSV) have developed formulation containing natural ingredients viz Curcumin, Vitamin C, Vitamin K2-7 and L-Selenomethionine.
Objective: To evaluate role of Curvic TM in the management of COVID-19 and its tolerability in comparison with standard treatment available from Ministry of Health and Family Welfare (MOHFW).
Study design and methodology: In a randomized, two arm, comparative study, COVID-19 positive patients (n=200) were enrolled during the month of August and September 2020 from Radiant Plus Hospital, Nashik, Maharashtra, India. The enrolled patients were administered either Curvic TM 500 mg tablets twice daily or given standard treatment protocol designed by MOHFW for 10 days. The patients were evaluated for decrease in oral temperature; SpO2 and VAS score for cough and respiratory distress. The underlying mechanism was assessed by evaluating markers such as Interleukin-6, Homocysteine, D-dimer, Ferritin and C Reactive Protein.
Results: In the Curvic TM group, within two days, temperature reduced considerably to afebrile level in all the subjects and remained afebrile till end of study. While in the standard treatment group, temperature reduced to afebrile stage in all the subjects by 4 days and there after remained afebrile till end of study. The elevated levels of serum Interleukin-6, Homocysteine, Di-dimer, Ferritin and C-Reactive Protein at baseline dropped considerably within normal limits within 10 days in the Curvic TM group in comparison to standard treatment group.
Conclusion: Curvic TM has shown for the first time to be useful in management of COVID-19 positive patients along with improvement in the immunity, within 48 hours of starting treatment. Curvic TM was well tolerated without any side effects in any of the patients treated.
Curcumin; COVID-19; IL-6; Homocysteine; SARS-CoV
Coronaviruses (CoV) are the family of viruses that are known to cause common cold, Middle East Respiratory Syndrome (MERS- CoV) as well as Severe Acute Respiratory Syndrome (SARS-CoV) [1]. During last two decades, as many as three novel human COV originated from animals. These were MERS-CoV, SARS-CoV and COVID-19 [2]. These pathogenic strains of COV have led to healthcare emergencies and have caused huge morbidity and mortality [3]. In year 2003, in a major SARS-CoV outbreak over 8000 individuals got infected across the globe with 9.6% fatality rate. It resulted in huge stress and loses on the global economy that in the range of US $100 billion [4,5]. Since the identification of first patient with severe pneumonia in Wuhan, China, COVID-19 has spread across the globe at a rapid pace causing the greatest pandemic of modern era. The estimated impact on global economy is over US$ 1 trillion [6]. Its rapid spread with mortality estimates of 1%-5% forced WHO to declare Public Health emergency [7]. The virus majorly spreads through coughing and sneezing. It is reported to be more infectious when patients are symptomatic. It is estimated that 1 out of every 6 COVID-19 patients may become seriously ill and develop breathing difficulties. Older people, and those with co-morbidities like diabetes, hypertension or cardiac problems, may develop serious illness [8].
At the moment, there is no specific/designated treatment for COVID-19. Exponential and continuous rise in the number of COVID infected cases has caused unusual acute unmet medical need. Researchers across the globe are working on number of treatment as well as preventive strategies to stop the spread of virus and to cure infected patients. Countries have put war scale efforts to develop vaccine. Researchers are exploring benefits of plasma therapy, interferon based treatments, monoclonal anti bodies, products from plants and herbal origin, as well as they are evaluating options of repurposing of approved drugs [9].
SARS-CoV-2 consisted of four structural proteins, like spike protein-S, envelope protein-E, membrane protein-M, and nucleocapsid proteins-N [10]. The Mpro protease is the major protein needed for the proteolytic maturation of the virus and its inhibition would inhibit viral replication. Thus, focusing and developing Mpro protease inhibitors can provide effective treatment against SARS-CoV-2 by inhibition of the viral polypeptide cleavage [11].
Herbal extracts and compounds of natural origin are used in treatment of various diseases since centuries. Curcumin, the powerful natural antioxidant is a yellow polyphenolic compound derived from the rhizome Curcuma longa [12]. Over the past few years, curcumin and its reductive metabolites have attracted interest of researchers due to its pleiotropic potential. It is reported to have wide range of pharmacological activities by modulating various cellular and molecular pathways, like COX-2, MMPs, glutathione, protein kinase C, ATPase, nuclear NF-kb, AP-1,P-gp, MRP-1, MRP- 2, ErbB2, a1- acidglycoprotein, Cyclin D1, and others [13,14]. Previous reports suggested that it directly interact with as much as 30 proteins but not limiting to polymerase, thioredoxin reductase, Focal Adhesion Kinase (FAK), Protein Kinase (PK), tubulin, and Lipoxygenase (LOX). It can incur post transcriptional and post translational changes and interfere with critical steps of viral replications and viral attachment. The bio-activities of curcumin are ascribed to its metabolites such as dimethoxycurcumin and tetrahydrocurcumin. Recently molecular docking studies suggested that COVID-19 Mpro was docked with dimethoxycurcumin which appeared to have the best potential to act as COVID-19 Mpro inhibitors [15]. There are no specific data available on effects of tetrahydrocurcumin against COVID-19 infection. Researchers at SSV used tetrahydrocurcumin against COVID-19 as one of the major compound (Patent number–IN202021013430) and showed for the first time, the promising antiviral effect which inhibited SARS-CoV’s main peptide (binding energy of -8.08 and inhibition constant of 1.2 μM) and COVID-19 Mpro (binding energy of -7.67 and inhibition constant of 2.32 μM) [15].
Vitamin K2-7 consumption is known to be beneficial for health. It is reported to have strong anti-inflammatroy activities due to its high bioavailability [16]. There are reports which suggest severity of COVID-19 disease and its comorbidities could be linked to its deficiencies. Dound et al. developed Vitamin K2-7 containing more than 98.5% trans isomers and less than 0.2% cis-isomers and found that vitamin K2-7 exhibits antiviral activity against HIV and HBV (Patent number–IN202021007016). They also evaluated in silico, the activity of vitamin K2-7 against SARS-CoV’s main peptide and COVID-19 Mpro (Patent number– IN202021013430). It was reported that Vitamin K2-7 has activity against SARSCoV’s main peptide (binding energy of -6.92 and inhibition constant of 8.48 μM) and COVID-19 Mpro (binding energy of -6.54 and inhibition constant of 16.24 μM), suggesting its potential role in prevention of COVID-19 [15]. Vitamin C and Selenomethionine are known to be very strong anti-oxidant, with immune boosting and anti-inflammatory activities. Selenium insufficiency is reported to increase severity and has been suggested to contribute in “the emergence of novel viral diseases”. Selenium deficiency causes impaired glutathione peroxidase production further leading to lung inflammations more so during viral infections. Some reports suggest selenium supplementation may lower the risk of corona virus infection [17-20].
Keeping scientific rationale, outcome of in silico studies and the therapeutic potential of Curcumin, Vitamin C, Vitamin K2-7 and L-Selenomethionine in view, researchers from SSV for the first time developed a formulation “Curvic TM” with these four ingredients for the management of COVID-19 positive patients. The objective of the current study was to evaluate clinical efficacy and safety of Curvic TM in COVID-19 infected patients.
Study design
This was a randomized, two arm, comparative study to assess the role of Curvic TM in the management of COVID-19 positive patients in comparison with standard treatment protocol designed by MOHFW. This study was conducted at Radiant Plus Hospital, Nashik, Maharashtra, India. The Study Protocol was approved by Navsanjeevani Hospital Ethics Committee and registered with CTRI (CTRI/2020/07/026839). Written informed consent was obtained from all patients.
Study participants
Among the two thirty patients screened, two hundred eligible patients meeting inclusion and exclusion criteria were randomized to receive either Curvic TM (Active group) or standard treatment protocol designed by MOHFW (Standard group) for 10 days. The random sequence was generated based on computer generated randomization.
Inclusion criteria
Patients between the age of 18-65 years, Quarantined/non hospitalized or hospitalized and fulfils WHO case definition, including a positive RT-PCR confirmed COVID-19 illness, classified as mild to moderately COVID-19 disease (NEWS score less than or equal to 8), with oxygen saturation (SpO2) less than 95%, Respiratory rate is more than 20/min, Pulse rate more than 90/min, Imaging evidence of lung infection in the form of reticulonodular opacities, ground-glass opacities and consolidation were enrolled in the study.
Exclusion criteria
Pregnant and Breastfeeding women, asymptomatic patients and those requiring ICU admission at screening, patients with past history of MI, epileptic episodes and any other co-morbidity (uncontrolled diabetes, severe hypertension from subject history), patients with prolonged QTc interval on ECG were excluded from the study. Patients with any immunosuppressive condition or haematological disease and in the opinion of the clinical team, progression to death is imminent and inevitable within the next 24 hours, irrespective of the provision of treatments were also excluded from the study.
Patients meeting the eligibility criteria and after written informed consent process, their medical history was recorded in Case Record Forms and physical examination was performed. For efficacy and safety evaluation various lab investigations were performed at different time intervals as per the approved protocol at central pathology lab accredited by NABL. The investigations included CBC, ESR, PT, renal function tests, Liver Profile, Homocysteine, Pro inflammatory cytokine IL-6, D-Dimer, Ferritin and CRP. The subjects were examined daily for symptoms which include cough, fever with or without chills and difficulty in breathing.
Study outcome
The primary outcome included clinical status as decided by the Principal Investigator based on the physical examination, laboratory investigations and signs such as oral temperature, respiratory rate, pulse, blood pressure and symptoms which include cough, fever with or without chills and difficulty in breathing. Changes in cough VAS score for cough and respiratory distress were used as a surrogate marker for response to treatment which has a scale from 0–10 cm with 10 cm being the worst imaginable cough.
Patients were assessed once daily by trained nurses using cards that captured data on a six-category ordinal scale and safety from day 0 to 10. Clinical improvement was defined as a two-point reduction inpatients’ admission status on a six-category ordinal scale (from 1=discharged to 6=death), or live discharge from the hospital, whichever came first. A special WHO committee arrived at this ordinal scale which measures illness severity over time. The clinical status was assessed based on the symptoms at baseline and at the end point along with parameters including homocysteine, Pro inflammatory cytokine IL-6, D-Dimer, Ferritin and CRP.
Study intervention
The study patients were randomized into two groups, patient in one arm randomly received Curvic TM tablets and patient randomized in the other arm received Ministry of Health and Family Welfare (MOHFW) recommended standard treatment. The formulation tablets, i.e. Curvic TM were manufactured and supplied by SSV in the form of tablets of 500 mg each packed as 30 tablets per bottle. The tablets were supplied in bottles to patients at the time of the enrolment. Patient consumed each tablet orally twice a day immediately after breakfast and after dinner for 10 days. The tablets compliance was judged by counting the number of tablets in the bottle left at the end of study. Patient was said to be compliant if he had consumed minimum 80% of the total dispensed tablets in Figure 1.
Figure 1: Study flow chart.
Statistical analysis
Data was presented as descriptive statistics such as mean ± standard deviation. Various statistical tests were used for the statistical analysis which include pair t test, ANOVA, Chi-squared test, Wilcoxon rank-sum test etc. Categorical data were presented as frequency and percentage. Statistical Analysis was done by an independent statistician using SPSS software.
All the 200 patients enrolled in the study completed their study. All the patients had complaints of cough, fever without chills and difficulty in breathing. Out of 200 patients, 100 patients were randomized to receive Curvic TM (Active group) while 100 patients received standard Treatment protocol designed by MOHFW (Control group). In the Active group there were 70 males and 30 females, while in the Standard group there were 76 males and 24 females. The average age of the study population in active group and control group was 42.33 years and 41.45 years respectively. There was no significant difference in the average age of study population in two groups. Majority of the study population (72%) was between the age 31 to 60 years in Table 1.
| Control | Active | t-value | p-value | |
|---|---|---|---|---|
| Age (years) | 41.45 ± 10.68 | 42.33 ± 13.42 | .513 | .609 |
Data are expressed as mean ± SD
Table 1: Demography-Age.
SpO2 levels
In the control group, mean baseline SpO2 levels were 83.50 and in the active group, mean SpO2 levels were 83.28. After the respective treatments, SpO2 levels were significantly improved day 2 onwards. In active, the increase in mean SpO2 levels were 13.2% on day 2, whereas in the control group the increase in SpO2 levels were 3.6% only. The increase in mean SpO2 levels in active group and control group were 17% on day 10. The Improvement in SpO2 levels were statistically significant (p=0.0001).
The improvement in SpO2 levels between different age groups were also compared. The study data was divided into different age wise groups. i) below the age of 30 years, ii) 31-40 years, iii) 41-50 years, and iv) above the age of 51 years. In all the age groups, the improvement in SpO2 levels in active group were statistically highly significant (p=0.0001) as compared to control group on day 2 and day 4. Younger population below the age of 30 years and older population above the age 50 years also responded better to active group than control group on day 10 and SpO2 levels enhancement was statistically significant.
In Active group, 100% of study population showed significant improvement in SpO2 levels on day 2 where 94% of study population showed mild improvement in SpO2 levels in Figure 2.
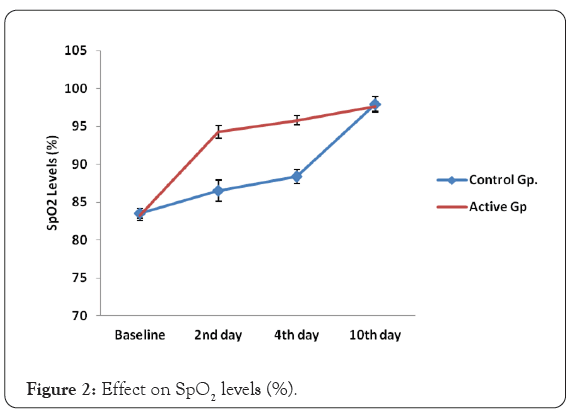
Figure 2: Effect on SpO2 levels (%).
Oral temperature
In the Active group and control group, the average temperature at baseline was 101 °F. In the active group, by the end of 48 hours, the average oral temperature reduced to 98.43 °F. The temperature continued to remain afebrile till day 10. In the Control group, by the end of 48 hours, the average oral temperature was around 100 °F and it reduced to 98.46 on day 4. These patients continued to remain afebrile till day 10 from 4-5 days onwards. The decrease in average oral temperature was statistically significant (p=0.0001). Active group showed significant reduction (p=0.0001) in oral temperature as compared to control group on day 2 and day 4.
Patients in all the age groups showed significant improvement (p=0.0001) in oral temperature in active group as compared to control group on day 2 and day 4.
In control group, on day 2, 94% of the patients showed decrease in oral temperature, 1% showed no change and 4% showed increase in the oral temperature, whereas in active group, all the subjects reached to afebrile condition on 2nd day itself in Figure 3.
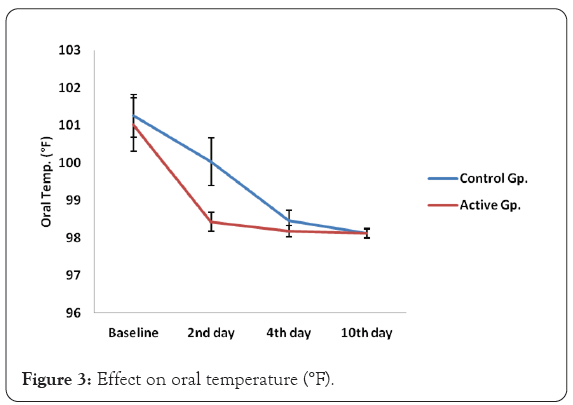
Figure 3: Effect on oral temperature (°F).
VAS score
The average VAS score for the cough and respiratory distress in both the groups was 6-7. By Day 2, the VAS score reduced to 3-4 in the Active group and 4-5 in the Standard group. By the end of Day 4, the VAS score further reduced to 0-1 in the Active group, while it remained at 4-5 in the Standard group. The score continued to remain at 0-1 in the Active group (Figure 4) while the score reduced to 3-4 in the Standard group (Figure 5) by the end of study i.e. day 10. The decrease in VAS score was statistically significant within the group also and between the group, on day 2, day 4 and day 10 (p=0.0001).
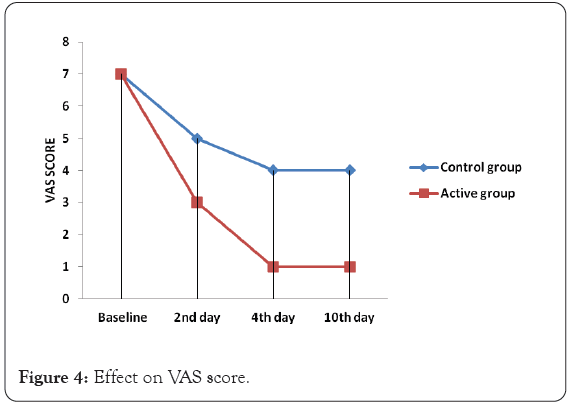
Figure 4: Effect on VAS score.
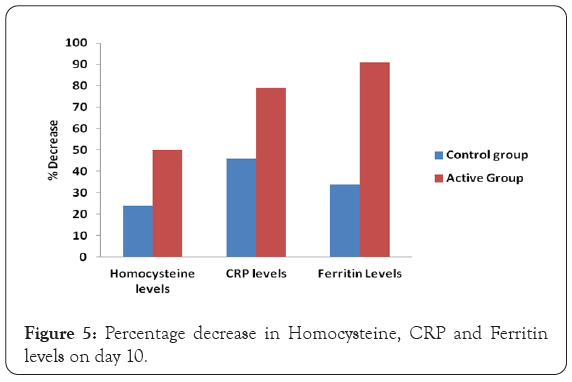
Figure 5: Percentage decrease in Homocysteine, CRP and Ferritin levels on day 10.
In all the different age groups, on day 2, day 4 and day 10, the reduction in VAS was more and significant in active group as compared to control group.
Six point ordinal scale
The patients in both the groups were in category 3 of the sixcategory ordinal scale of clinical status at baseline. Category 3 refers to the patients who are hospitalized with mild to moderate disease. The subjects in the active group were in the category 0 of this scale by day 10. The subjects in the standard group were in the category 1 of this scale by day 10. Category 0 refers to the patients who are not infected with no clinical or virological evidence of infection. Category 1 refers to the patients who are ambulatory with no limitation of activities. The standard treatment group could not achieve category 0 on this scale by day 10. The decrease in assessment by the 6 point ordinal scale in active group was significant in active group as compared to control group (P=0.0001) in Tables 2 and 3.
| Control | Active | t-value | p-value | |
|---|---|---|---|---|
| IL-6 Before | 1215.21 ± 153.29 | 1209.73 ± 162.05 | .246 | .806 |
| IL-6 After | 605.33 ± 85.75 | 38.66 ± 3.60 | 66.023 | .0001** |
**Data are expressed as mean ± SD
Table 2: Effect on IL-6 levels (pg/ml).
| Control | Active | Mann-Whitney U (z) | p-value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Median | Quartile-1 | Quartile-3 | N | Median | Quartile-1 | Quartile-3 | |||
| 6-point Ordinal Scale Before | 100 | 3.00 | 3.00 | 3.00 | 100 | 3.00 | 3.00 | 3.00 | 0.000 | 1.000 |
| After | 100 | 1.00 | 1.00 | 1.00 | 100 | 0.00 | 0.00 | 0.00 | 14.107 | .0001** |
** Data are expressed as mean ± SD
Table 3: Six point ordinal scale.
Clinical assessment
Serum IL-6 levels: The mean IL-6 levels were reduced significantly on day 10 in both the groups (p=0.0001) as compared to respective baseline levels. The reduction in control group was 50% and in case of active group IL-6 levels reduced by 96%. The reduction in mean IL-6 levels in active group was highly significant (p=0.0001) as compared to standard group on day 10.
Serum homocysteine levels
The mean serum homocysteine levels (μmol/L) were reduced significantly on day 10 in respective male and female populations of both the active and control groups (p=0.0001) as compared to respective baseline levels respectively. The reduction in means serum homocysteine levels in control group was 24% and in case of active group serum homocysteine levels reduced by 50%. The reduction in mean serum homocysteine levels in active group was highly significant (p=0.0001) as compared to standard group on day 10.
CRP levels
The mean CRP levels were reduced significantly on day 10 in both the groups (p=0.0001) as compared to respective baseline levels. The reduction in control group was about 46% and in case of active group CRP levels reduced by 79% as compared to respective baseline levels. The reduction in mean CRP levels in active group was highly significant (p=0.0001) as compared to standard group on day 10. The decrease was 68% more in active group as compared to control group at the end of the treatment.
Serum ferritin levels
The mean ferritin levels were reduced significantly on day 10 in both the groups (p=0.0001) as compared to respective baseline levels. The reduction in control group was about 34% and in case of active group ferritin levels reduced by 91% as compared to respective baseline levels. The reduction in mean ferritin levels in active group was highly significant (p=0.0001) as compared to standard group on day 10. The decrease was 86% more in active group as compared to control group at the end of the treatment in Table 4.
| Control | Active | t-value | p-value | |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | |||
| Ferritin Before | 555.08 ± 94.17 | 568.32 ± 75.45 | 1.098 | .274 |
| Ferritin After | 367.09 ± 33.01 | 51.07 ± 8.10 | 92.974 | .0001** |
**Data are expressed as mean ± SD
Table 4: Effect on serum ferritin levels (μg/L).
D-Dimer levels
The mean D-Dimer levels were reduced significantly on day 10 in both the groups (p=0.0001) as compared to respective baseline levels. The reduction in active group was greater and significant (p=0.0001) as compared to control group. The reduction in mean D-Dimer levels in active group was highly significant (p=0.0001) as compared to standard group on day 10. The decrease was 74% more in active group as compared to control group at the end of the treatment in Table 5.
| Control Mean ± SD |
Active Mean ± SD |
t-value | p-value | |
|---|---|---|---|---|
| D-Dimer Before | 2.42 ± .25 | 2.27 ± .22 | 4.370 | .0001** |
| D-Dimer After | 1.44 ± .27 | .38 ± .09 | 37.649 | .0001** |
**Data are expressed as mean ± SD
Table 5: Effect on serum D-Dimer levels (mg/L).
Safety evaluation
No significant change in haematological parameters, liver functions tests and renal function tests were observed in both control and active group. TThere was no decrease in body weight of the subjects in the Active group, while there was decrease in body weight of many subjects in the Control group. Among these patients there were some who were on oral medications for hypertension and Type 2 diabetes mellitus. These patients continued with their regular medications along with Curvic TM. It was observed that there was no drug interaction of Curvic TM with any of these medications.
In the current study, the formulation combining the ingredients viz Liposomal Curcumin, Vitamin C, Vitamin K2-7 and L-Selenomethionine were evaluated for the management of COVID-19 and to also to improve the immunity. There are many reports which clearly suggest antiviral potential of curcumin. Its antiviral activity was reported for parainfluenza virus type 3, herpes simplex virus, flock house virus and respiratory syncytial virus [21]. It is reported to trigger cellular signaling pathways like apoptosis. It is also reported to modulate NF-κB and PI3K/Akt signaling which is reported to be critical for viral replication. Curcumin is reported to have multi directional impact, it reported to alter surface proteins in viruses, thus inhibiting it entry and budding. It is also reported to have modulating effect of membrane proteins by altering host lipid bilayer characteristics. It impedes viral infection by inhibiting viral penetration as well as impacting factors required for virus replication [16]. Our molecular docking studies has shown that Curcumin esp, Tetrahydrocurcumin binds of the viral Mpro protease, which is responsible for viral replication and maturation.
Curcumin has anti-inflammatory and anti-fibrotic effects by reducing the expression of crucial chemokines and cytokines involved in lung infection such as IFNγ, MCP-1, IL-6 and IL-10. Researchers also studied effect of curcumin on pulmonary fibrosis and pulmonary oedema [22]. It is reported to inhibit apoptosis pathway and TGF-ß pathway thus reducing pulmonary fibrosis [23]. Gausal and Sarada reported that curcumin down regulated pro-inflammatory cytokines, angiogenic molecules like VEGF leading to decrease in pulmonary oedema [24].
Vitamin C, a free radical scavenger, powerful antioxidant and an essential micronutrient was recommended as a non-specific treatment for severe viral lung infections [25]. It is reported to support innate and adaptive immune system along with decreasing inflammations. COVID-19 linked respiratory dysfunction is associated with immune hyper reaction and IL-6 and ET-1 play crucial role [26]. Vitamin C is reported to reduce these inflammatory mediators thus reducing tissue damage. It is reported to inhibit production of IL-6 and Tumor Necrosis Factor alpha (TNF-α). It is reported to regulate proliferation and functioning of B cells, T cells and natural killer cells. This could be contributing in the inhibition of progression of cytokine storm and improving hosts immune response [27].
Vitamin K2-7 belonging to Vitamin K group has shown to be effective in reducing pro inflammatory cytokines. A recent preliminary research conducted by Dofferhoff ASM et al. has shown that low Vitamin K status may worsen outcomes for COVID-19 [28]. For the first time it has been shown that Vitamin K2-7 effectively binds the viral Mpro protease, which is responsible for viral replication and maturation. Selenium is known to have strong antioxidant activity and is reported to alter spike proteins thus inhibiting entry of the virus [29]. It is also reported to have synergistic effect with Vitamin D, it improves immune system by enhancing proliferation of natural killer cells and down regulating IL-6. It is also reported to inhibit clot formation which is one of the major causes of mortality in COVID-19 patients [30]. Prior to current study, a pilot clinical study was conducted on quarantined 30 COVID patients (CTRI/2020/04/024659) by the same group of researchers and the patient response was very encouraging. Most of the patients (97%) showed some kind of improvement in fever and related symptoms within 48 hours of treatment of treatment with Curvic TM. Keeping the positive outcome of the pilot study, authors planned to conduct a study on larger patient population of COVID-19 (n=200).
In the current study, a significant improvement in oral temperature, SpO2 levels, VAS, 6 point ordinal scale were observed in active group and control group suggesting patients were relieved of viral infection and became a symptomatic. Patients treated with Curvic TM (active group) recovered faster from the COVID-19 infection as they became afebrile on the 2nd day itself. In addition, the patients in active group started recovering earlier than control group as indicated by significant decrease in score of VAS, SpO2 and 6 point ordinal scale.
Clinicians use CRP, D-dimers, Ferritin, Homocysteine and IL-6 levels to predict severity of the COVID-19 infection. IL-6 a proiflammatory cytokine, is considered to be one of the important therapeutic targets. In the current study, the mean IL-6 levels were reduced significantly on day 10 in both the groups (p=0.0001) as compared to respective baseline levels. The reduction in control group was 50% and in case of active group IL-6 levels reduced by 96%. The reduction in mean IL-6 levels in active group was highly significant (p=0.0001) as compared to standard group on day 10. Homocysteine is a non-proteinogenic amino acid and elevated homocysteine concentrations are shown to be associated with immune system activation and increased neopterin serum concentrations. The homocysteine levels are suggested as a potential marker for severity of the disease in COVID-19 patients [31]. In our study, the decrease in means serum homocysteine levels in control group was 24% and in case of active group serum homocysteine levels reduced by 50%. The reduction in mean serum homocysteine levels in active group was highly significant (p=0.0001) as compared to standard group on day 10. Ferritin is a prime mediator of immune dysfunction, especially during hyper-ferritinemia, because of its immuno-suppressive and pro-inflammatory impact, leading to the cytokine storm [32]. Laboratory investigations in the patients with severe COVID-19 showed data consistent with cytokine storm involving elevated inflammatory markers, including ferritin, which has been associated with critical and life-threatening illness [33]. The mean ferritin levels were reduced significantly on day 10 in both the groups (p=0.0001) as compared to respective baseline levels. The reduction in control group was about 34% and in case of active group ferritin levels reduced by 91% as compared to respective baseline levels. The reduction in mean ferritin levels in active group was highly significant (p=0.0001) as compared to standard group on day 10. CRP levels are correlated with the level of inflammation. CRP levels activate the complement and enhance phagocytosis, thus clearing the pathogenic microorganisms which invade the body. It is an important diagnostic marker for inflammation and infections [34]. The elevated levels of CRP are linked to the overproduction of inflammatory cytokines in severe patients with COVID-19 [35]. Higher levels of CRP can be considered as a predictive marker in determining which patients with mild coronavirus disease 2019 (COVID-19) will progress to a severe case, according to study results published in an open forum infectious diseases. In this particular research study, the reduction in mean CRP levels in active group was highly significant (p=0.0001) as compared to standard group on day 10. The decrease was 68% more in active group as compared to control group at the end of the treatment. D-dimers are one of the fragments produced when plasmin cleaves fibrin to break down clots. However, physiologic process that enhances fibrin production or breakdown leads to increase in plasma D-dimer levels. D-dimer concentrations might be helpful to rapidly identify COVID-19 patients with high risk of pulmonary complications and venous thromboembolism, facilitating the early initiation of effective therapies [36,37]. The mean D-Dimer levels were reduced significantly on day 10 in both the groups (p=0.0001) as compared to respective baseline levels. The reduction in active group was greater and significant (p=0.0001) as compared to control group.
The significant decrease in these disease severity predictive markers and improvement in COVID-19 infected patient’s condition on treatment with Curvic TM and early recovery of the patients as compared to standard treatment suggests that Curvic TM better managed COVID-19 patients as compared standard treatment regimen.
Curvic TM formulation, consisting of liposomal curcumin, vitamin C, K2-7 and Selenomethionine reduced inflammatory markers, improved patients disease condition to asymptomatic with complete relief from symptoms could be developed into a valid therapeutic option.
Authors are thankful to M/s Shreepad Shree Vallabh SSV Phytopharmaceuticals for providing study supplies and financial support.
Citation: Dound YA, Suryavanshi S, Sehgal R, Naik A (2020) Comparative Clinical Study to Evaluate the Activity of Curvic TM Formulation for Management of SARS-CoV-2 Infection (COVID-19). J Clin Trials. S3:004.
Received: 22-Oct-2020 Accepted: 09-Nov-2020 Published: 16-Nov-2020
Copyright: © 2020 Dound YA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.