
Journal of Clinical Trials
Open Access
ISSN: 2167-0870

ISSN: 2167-0870
Research Article - (2023)Volume 13, Issue 5
Background: Evidence suggests patients with type 2 diabetes who smoke are at increased risk of developing health complications and premature death. Incorporating tobacco cessation intervention within regular care for people with diabetes would provide a valuable opportunity to support this vulnerable group quitting combustible tobacco. A novel category of reduced-risk tobacco products, Oral Nicotine Pouches (ONPs), has recently emerged. There is no evidence on how effective ONPs are for smoking cessation among people with pre-existing chronic health conditions, like diabetes. Globally, Bangladesh is one of the top ten countries with the highest adult smoking rates and the number of adults with diabetes. Therefore, this clinical trial will assess the long-term health effects of smoking cessation through an ONP intervention among type 2 diabetic patients in Bangladesh.
Methods: The study is a two-arm Randomized Control Trial (RCT) to be conducted in three phases: A 12-week intervention, a four-week phase-out, and a follow-up assessment at Month-6, Month-9, and Month-12. Type 2 diabetic patients (n=440) recruited from a clinical setting will be randomized to either the intervention or the control group (1:1 ratio). The intervention group will receive 6 mg dry ONPs over 12 weeks, with tapering doses for the next 4 weeks (3 mg for 2 weeks and 0 mg for 2 weeks). The control group will be in usual diabetic care for the entire study period. The primary study outcomes measures include 7-day point prevalence smoking abstinence validated by expired-air carbon monoxide at week-12, and clinical outcomes (pulmonary, liver, and kidney functions; inflammatory biomarker, body composition, blood glucose and HbAc1, blood pressure, and lipid profile) at week- 12 and week-52. Secondary outcomes are changes in cigarette consumption, side-effects, use, acceptability, and reasons for using ONPs; use of other tobacco, nicotine products, and smoking cessation methods; and suitability of intervention.
Discussion: This RCT will provide strong evidence to determine the health impact of a tobacco harm reduction product and valuable insights into the overall potential of smoking cessation in reducing complications in patients with diabetes and significantly contribute to policy decisions.
Diabetes; Type 2; Oral nicotine pouches; Randomized Control Trial (RCT); Smoking cessation; Intervention; Clinical outcomes; Long-term impact
The survey instruments that will be administered in this study largely comprise measures including questions and response options extracted or adapted from peer-reviewed validated questionnaires that have been used in previous national surveys and individual research for assessing tobacco use behaviors and related issues among adult participants. However, measures are tested with the Bangladeshi type 2 diabetic patients.
Pretesting
Smoking cessation research has not been expanded widely in Bangladesh; all internationally validated measures may not have been used with Bangladeshi participants in the past. As a result, it is important to test the questionnaires in a clinical setting with diabetic patients to understand the local and cultural context. A pre-testing session was run with a small group of smokers with type 2 diabetes (n=16) visiting EMCH in March 2023 to understand the research instruments developed from validated measures for the proposed clinical trial to be appropriate, acceptable, and understandable among the targeted respondents in a Bangladeshi setting. In addition, the recruitment process was tested at the Department of Endocrinology of EMCH. The trial design addressed and used participants’ and interviewers’ feedback in refining the recruitment procedures and data collection instruments. Data collection measures and timelines are summarized in Table 3.
| Theme | Measure | Test/questionnaire | Screening | Baseline | During intervention | End of intervention, 3M | Phase-out | Follow-up -6, 9-,-12M |
|---|---|---|---|---|---|---|---|---|
| Inclusion | Screening for inclusion | Screener questions | X | |||||
| Exhaled CO | CO breath test monitor | X | X | |||||
| Availability for the study | Screener questions | |||||||
| Signature of informed consent | Consent form | |||||||
| Physical measures | Weight | Bathroom scale | X | X | X | |||
| Health | Height board | X | X | X | ||||
| BMI | Height and Weight ratio | X | X | X | ||||
| Heart rate | Pulse at wrist | X | X | X | ||||
| Blood pressure | Sphygmomanometer | X | X | X | ||||
| Clinical measures | Blood glucose | Fasting blood | X | X | X | |||
| HbA1c | Fasting blood | X | X | X | ||||
| Lipid profile | Fasting blood | X | X | X | ||||
| C-reactive protein | Fasting blood | X | X | X | ||||
| Serum Glutamic Pyruvic Transaminase (SGPT) | Fasting blood | X | X | X | ||||
| Serum Glutamic-Oxaloacetic Transaminase (SGOT) | Fasting blood | X | X | X | ||||
| Creatinine | Fasting blood | X | X | X | ||||
| Pulmonary function | Spirometry | X | X | X | ||||
| Co-morbidities | Co-morbidities | Medical record | X | X | X | |||
| Medication | Medical record | X | X | X | ||||
| Demographics | Sociodemographic data | Validated questions | X | |||||
| Smoking | History | Validated questions | X | |||||
| Current smoking | X | X | ||||||
| Other tobacco products | History | Validated question | X | |||||
| Current use | Validated questions | X | X | X | X | X | ||
| Healthcare support to stop smoking | Visit to doctors and advice received | Validated question | X | |||||
| Quit attempt | Quit attempts | Validated question | X | X | X | X | X | |
| Method used to quit | Validated question | X | X | X | X | X | ||
| Intention to quit | Motivation to stop smoking in the next 6 months | Validated question | X | |||||
| Co-morbidities | Current comorbid conditions | Self-administered co-morbidity questionnaire (SCQ) | X | X | X | |||
| Quality of Life | Health-related quality of life | Short form-12 (SF-12) | X | X | X | |||
| Flavor | Flavor preference after testing | Validated question | X | X | X | |||
| Reasons for interest in using | Endorse the main reason for interest in using pouches | Validated question | X | |||||
| Abstinence | Smoking Abstinence in the past 7 days | Validated question | X | X | ||||
| Medication change | Alteration of regular medications for diabetes after initiation of ONPs use. | Validated question | X | X | ||||
| Change is disease conditions | Changes in health-related issues experienced after initiation of ONPs use. | Validated questions | X | X | ||||
| Change in physiologic functions | Changes in physiologic functions after OPNs use initiation | Validated questions | X | X | ||||
| Intention to continue | Interest in using the product again | Validated scale | X | X | ||||
| Acceptance | Product liking | Validated scale | X | |||||
| Reasons for use | Why still using ONPs | Validated question | X | |||||
| Reasons for not to use | Why stopped using ONPs | Validated question | X | |||||
| Use and acceptability | No. of used and unused pouches in the past 7 days | Daily diary | X | X | X | |||
| Side effects of ONPs | Any side effects in the past 7 days | Validated Questions | X | X | X | |||
| Daily smoking | No. cigarettes smoked each day | Daily diary | X | X | X | X | ||
| Daily ONPs | No. pouches used each day | Daily diary | X | X | X | |||
| Stop smoking advice | Individual face-to-face counselling (control group) | Behavioural Counselling | X | |||||
| Acceptance and suitability | Qualitative Interviews (Intervention participants) | Qualitative Topic Guide | X | |||||
| Qualitative Interviews (Research Staff) | X |
Table 3: Data collection measures and data collection points
Demographic characteristics
Demographic information, including sex, age, marital status, education, employment, and income, will be recorded.
Current smoking
Questions will assess participants’ ever-smoking status, past 30 days of smoking, current smoking status, type of tobacco use (cigarettes or bidis), the average number of cigarettes or bidis smoked per day now, and time since initiation of regular smoking.
Smoking history
Questions will ask participants about the age of first-ever tried smoking and the age at started smoking regularly.
Smoking abstinence
Smoking abstinence will be self-reported and validated by exhaled CO level measurements with a calibrated handheld monitoring device at hospital visits. Abstinence from smoking will be assessed by checking the 7-day daily diary, in which participants will keep a record of the number of cigarettes they have smoked daily in the past 7 days. Point-prevalence abstinence will be defined as those who self-reported smoking 0 cigarettes in the past 7 days in the diary verified by a CO level<10 ppm. Continuous abstinence will be considered if participants have reported not having smoked cigarettes since the quit day reported during the intervention and have an expired CO reading of<10 ppm. If there is a discrepancy between the participant report and CO level, the subject will be coded as self-reported abstinence for that visit. Using a single question, self-reported abstinence in the past 7 days will also be collected during follow-ups (Did you smoke tobacco during the past 7 days (even one puff of a cigarette or bidi)?). The cigarette smoking reduction or increase or no change will also be assessed in the participants who cannot achieve abstinence.
Health condition
Self-reported history of oral diseases and general physical and mental health status will be collected from the participants using questions.
Diabetes history and medication
Questions will assess the time since participants were diagnosed with diabetes and the type of treatment received currently, e.g., insulin or diabetic pills. The name of medicines and doses will be recorded from the patient’s hospital file.
Comorbidities
Self-administered Co-morbidity Questionnaire (SCQ) will be used to determine comorbidities and their impact on working performance and healthcare utilization [41]. The questionnaire lists 12 common comorbidities and three additional non-specified medical problems. Participants will be asked to indicate if they have the condition, if they receive treatment, and if the condition limits their activities. The medical conditions are heart disease, high blood pressure, lung disease, ulcer or stomach disease, kidney disease, liver disease, anemia or other blood diseases, cancer, depression, osteoarthritis, back pain, rheumatoid arthritis, and an option to add on other no-specified medical problems.
Attempt to quit smoking
At baseline, participants will be asked: (i) If they have tried to quit smoking altogether in the past 12 months and (ii) How long they have managed to stop smoking in the last quit attempt. Participants who report having attempted to quit smoking within the last 12 months will be asked about their use of different smoking methods (counseling, NRT, prescription medications, traditional medicines, electronic cigarettes, or cold turkey) used as part of their attempt to quit smoking in the last 12 months. As part of the weekly visit, participants will be asked if they have used stopping smoking products in the past 7 days, and in the monthly visit, participants will be asked if they have used methods to stop smoking in the past 30 days.
Use of other tobacco or nicotine products
At the baseline, questions will assess participants’ use of each of a list of other tobacco and nicotine products within each of the following timeframes, as applicable: (i) ever used; (ii) used in the past 30 days; and (iii) used in the past 7 days. During weekly home visits, participants will be asked to indicate which, if any, of a list of other tobacco and nicotine products they have used in the past 7 days, and during monthly home visits, participants will be asked to indicate which, if any, of a list of other tobacco and nicotine products they have used in the past 30 days and the past 7 days.
Intention to quit
Participants will be asked if they intend to quit smoking [42]. Response categories will be “not planning to quit”, “planning to quit sometime in the future, beyond 6 months”; “planning to quit within the next 6 months”, and “planning to quit within the next month”. Participants will also be able to answer with “don’t know”. Participants who will select ‘in the next month’, ‘in the next 6 months’, or ‘sometime in the future after 6 months’ will be defined as having an intention to quit, and those who responded ‘not at all’ will be defined as having no intention to quit.
Liking and intention to continue ONPs
At the end of the intervention, participants will be asked how much they like the ONPs using a product-liking question [43]. The answer will be given on a scale of 0 (strongly dislike) to 10 (strongly like). At the end of the intervention and follow-up period, participants will be asked a question on an intent-to-use-again with answers option on a scale of 0 (not at all) to 10 (very much) [43]. For both measures, the percentage of participants will be grouped into three categories: lowest (<5), middle (5), and higher (>5).
Use and acceptability of intervention
Use and acceptability of intervention (ONPs) will be determined by comparing the number of used and unused pouches returned at each weekly visit.
Quality of life
The 12-item Short Form (SF-12) will be used to assess health-related quality of life [44]. SF-12 consists of twelve questions on physical and mental health quality of life assessments. Scores range from 0 to 100, with higher scores indicating better physical and mental health functioning.
Support from health care providers
Participants will be asked if a healthcare provider has seen them in the past 12 months [15]. Those who report a visit in the last year will be asked the following questions: (i) the number of visits they have made in the last 12 months, (ii) whether they have been asked about smoking behavior, and (ii) whether they have been advised to quit smoking tobacco by their health care provider during any visit in the past 12 months. Those who received smoking cessation advice in the last year will be asked about the types of advice they have received to quit smoking tobacco from a doctor or health care provider the in the past 12 months.
Reasons for interest to use ONPs
When pouches are shown to the intervention group at the baseline, randomized intervention participants will be asked to select which, if any, of the 11 prespecified reasons provided explain why they are interested in using oral nicotine pouches over the next four months. Participants will be allowed three additional open-ended reasons besides the 11 predefined listed reasons.
Reasons for use and stopping use of ONPs
At the end of the intervention, participants who report the use of ONPs in the past 7 days in their 7 days diary will be asked to select their primary reason, which, if any, of a list of 20 reasons explaining why they currently use pouches. Four response options will be based on tobacco, including helping to reduce or quit cigarette smoking and/or other tobacco products. The rest of the response options will be related to various characteristics of the pouches (e.g., flavors, nicotine strength, and no spitting) and their impact on users and bystanders (e.g., less harmful and no smell). The list of response options is adapted from a study on oral nicotine pouches [45].
At the end of the intervention, participants who report no use of pouches in the past 7 days in the 7-day diary will be asked to select the main reason, which, if any, of a list of 19 reasons explain why they stopped using pouches.
Side effects after using ONPs
Side effects defined as any untoward occurrences among the participants as a perceived result of ONPs use, including a symptom or disease, will be assessed. Participants will be asked to report whether they have felt any adverse health effects over the previous week to during the intervention and phase-out using 19 side effects as response options related to nicotine delivery. Participants will be allowed to report additional side effects not listed through three additional open-ended response options.
Changes in physiologic functions
Intervention participants will be asked to rate their experience of any change (worse, no change, better) in 10 symptoms known to be positively impacted by THR products [46]: Smell, Taste, Breathing, Appetite, Sexual performance, Mood, Memory, Quality of sleep, Endurance and Physical status in general.
Changes in disease conditions after pouches use initiation
Intervention participants will be asked whether they suffered from any chronic health conditions before initiating pouches use. Those reporting any health condition will be asked to rate their experience of any change (worse, stable, improved) after initiating the use of pouches. Health conditions include Hypertension, Hypercholesterolemia, Thyroid disease, Coronary artery disease, Asthma, and Chronic Obstructive Lung Disease (COPD) [46]. Intervention participants will also be asked whether there has been any change in diabetic medications since they have started using pouches.
Physical and clinical measures
Physical measures will include height, weight, Body Mass Index (BMI) blood pressure, and heart rate. Fasting venous blood samples will be collected for analysis of Fasting blood glucose, HbA1C, Lipid profile (Cholesterol and Triglycerides), C-reactive protein, Serum Glutamic Pyruvic Transaminase (SGPT), Serum Glutamic- Oxaloacetic Transaminase (SGOT), and Creatinine.
Pulmonary Function Tests (PFT) will be measured using a spirometer. The highest level for Forced Vital Capacity (FVC), Forced Expiratory Volume in one second (FEV1), FEV1/FVC ratio, Peak Expiratory Flow (PEF), Estimated Lung Age (ELA), Forced Expiration Technique (FET), Forced Inspiratory Vital Capacity (FIVC), and Forced Expiratory Flow at 75%, 50%, and 25% (FEF25-75) will be taken independently. Self-reported cigarette smoking status will only be verified by exhaled CO levels using a Smokerlyzer.
Qualitative interview
At the end of the intervention, qualitative interviews will be conducted to explore the acceptability and suitability of the intervention and process of implementation in more depth among intervention participants and research staff. Fifteen participants and ten field research staff will be invited to take part in the interview. Participants and staff will be purposively selected to reflect diverse views, including abstainers and non-abstainers, varying by age and socio-economic status.
Confidentiality
The security of participants' collected data will be assured to the degree permitted by the protective technology. Electronic and paper data collected in this study will be secured and protected locally in accordance with its data privacy and General Data Protection Regulation (GDPR) policies.
Following the end of the study, electronic records of all de- identified study data and documents, including the final study report, instruments, and appendices, will be securely archived on a secure server and hard disk. Participants’ personal details will not be attached to the research results, and the decoding list will only be available to a limited number of research team members. All information obtained during the study procedures will be treated as private and confidential. Once data is genuinely anonymized and individuals are no longer identifiable, the data do not fall within the scope of the GDPR or destruction after the end of the study. However, anonymized data will be used for analysis publications for 5 years after the end of the study.
Statistical methods
Analyses of the primary outcome from the intervention will be done on an Intention-to-Treat (ITT) principle. ITT will consider all randomized participants in the analysis, whether they drop out or not. Analysis of secondary outcomes will follow the Complete-Case Analysis (CCA) technique, which will use only the data records without missing values for any variable needed for analysis.
Descriptive statistics will be presented in summary tables. Baseline and demographic data will be listed for two groups. Categorical data will be summarized by frequencies, proportions, and 95% CIs around proportions. The 95% CIs around proportions will be calculated using the Jeffreys interval method. Continuous data will be summarized by appropriate central tendency and dispersion measures for the observed data (e.g., mean with standard deviation, median with range). When applicable, questionnaire responses measured on a Likert scale will be analyzed following each instrument's scoring guidelines.
Pre- and post-intervention outcome variables will be described for each group. In controlled trials, randomization ensures that the allocation of participants to the intervention or control group is left purely to chance. Observed differences in outcomes between groups in a trial could occur by chance due to differences in the characteristics of the participants, not the intervention. However, to minimize the possibility of this bias, the results will be adjusted for baseline values [47]. Intervention and control group differences will be analyzed using two approaches: (a) unadjusted analysis, a direct comparison of the change in outcome measures. One-way Analysis of Variance (ANOVA) and Mann–Whitney U-test will be used to assess the significant difference between the two groups for normally and not normally distributed continuous variables, respectively; the χ2 test will be used for categorical variables. (b) Adjusted analysis, primary and secondary endpoints between the intervention and control group will be compared with baseline scores included in the models as covariates. Continued endpoints in the intervention and control groups will be compared using Analysis of Covariance (ANCOVA), and categorical endpoints will be compared using multiple regression models, with covariates included in each model as appropriate. Statistical significance difference between groups will be assessed at a two-sided p-value of 0.05. All data analyses will be conducted using IBM SPSS version 26 (or higher).
Handling of missing data
Missing data will commonly occur in the proposed trial, and data imputation will be applied to primary outcome variables. In those cases where it is not possible to locate the participants, they will be considered smokers and at the same level (in terms of smoking) as at the baseline assessment.
Data imputation will be employed to account for missing diary data on the number of cigarettes/bidis smoked daily. When data are missing, arbitrary rules, such as requiring at least 4/7 observations, will be used to determine whether a participant’s summary score is created or set to the baseline value. If the number of cigarettes/ bidis smoked by a participant is missing in his/her e-diary for three or fewer days of a study week, an average cigarettes/bidis per day will be calculated for the participant by dividing the total number of cigarettes/bidis consumed in that week by the number of days for which the participant recorded cigarettes/bidis consumption that week. The calculated average cigarettes/bidis per day will be imputed to each of the three or fewer cells with missing data in that study week.
If the number of cigarettes/bidis consumed by a participant is missing in the diary on four or more days of a study week, the participant’s average number of cigarettes/bidis reported in the last dairy will be imputed to each of the seven cells of the missing week (i.e., Last Observation Carried Forward (LOCF)).
If the number of pouches consumed by a participant is missing in his/her diary, the participant’s consumption of that pouches for the study week will be recorded from the supply of unused and used pouches for that week (i.e., subtracting the used pouches from the total number pouches delivered for that week).
For biological and clinical outcome variables, the LOCF method will be applied (the participant’s last observed biological and clinical value replaces a participant’s missing values). Where data points are missing on other outcome variables, the data will be assumed missing at random and data summaries will be based on reduced denominators that include only valid data.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
FH planned the study, developed the protocol, wrote the initial draft, and submitted the manuscript. TS and AMC helped plan the study and established the connection with the clinical site. GB provided input in designing survey tools and data collection plan. AH and NR contributed to the study's design and provided insight into the study's clinical aspects. AH and NR translated all the patient-facing study materials, and HR SI, AH, and NR tested the survey tools in a clinical setting. SR, AMC, SI, AH, and NR contributed to organizing patient and public involvement workshops. NM and JC developed the qualitative component of the study. All authors read and approved the manuscript for final publication. FH has the primary responsibility for the final content.
The contents, selection and presentation of facts, as well as any opinions expressed in the protocol are the sole responsibility of the authors and under no circumstances shall be regarded as reflecting the positions of the FSFW.
This protocol development was funded with a grant from the Foundation for a Smoke-Free World (FSFW) (Reference: FSFW W3-053), a US non-profit 501(c)(3) private foundation.
The authors declare that they have no competing interests.
The study will be conducted according to the principles of Good Clinical Practice (GCP), the Declaration of Helsinki, and local ethical guidelines. Before deciding whether to participate in this study, candidate participants will be asked to carefully review an ICF that will give written information about the purpose of the study, study procedures, requirements of participants within each part of the study, the potential health risks and benefits of participating, and compensation associated with participation. Candidate participants will be given sufficient time to read and consider the ICF presented the study and encouraged to ask any questions they may have about the study to the research team or consultants. Research staff will read and explain the ICF in case the respondent's literacy is insufficient to read and understand these documents. If a candidate expresses interest in participating in the study and signs the ICF, one copy of each signed ICF will be retained by the participant for his/her records, and one copy will be retained by the research staff and filed securely. The telephone number and contact address of the PI, RP, FRS, and study coordinator will be provided to the participants so that any queries they might have during the study period can be addressed in a timely manner.
In the event of an unusual health experience or discomfort after using ONPs, participants will be asked to stop using ONPs, and promptly report details of the side effects to the FRS by calling a mobile number available six days a week. If participants experience any severe side effects, participants will be asked to seek medical advice from a RP who will be available to receive phone calls. The RP will arrange the prompt diagnosis of any side-effect caused by or derived from the administration ONPs as soon as he/she becomes aware of it. Participants will be provided treatment under standard care if the side-effect is related to ONPs and needs treatment. In case of an emergency, participants will be advised to go immediately to the nearest hospital, and treatment costs will be reimbursed if related to ONPs.
This project will secure ethics approval from the Enam Medical College Ethics Committee and Bangladesh Medical Research Ethics Committee (BMRC). Two ethics committees will review the protocol and, where appropriate, translated relevant documentation (informed consent form, participant information sheet, questionnaire, and so on).
The proposed future study has the potential to shape knowledge and future research on the feasibility of using patient health care services to deliver smoking cessation to high-risk patient populations receiving treatments in hospitals or health centers. With the completion of the proposed research, it will be possible to inform clinical settings in Bangladesh as to the potential impact of providing the millions of diabetic adult smokers in Bangladesh with access to oral nicotine products as a way of facilitating their smoking cessation. The research will likely be generalizable to other clinical settings. Therefore, this research project will provide valuable insights into the overall potential of smoking cessation to reduce the risk of tobacco-related complications in patients with diabetes living in low- and middle-income countries and significantly contribute to policy decisions. If the current project is successful in Bangladesh, implementing a similar intervention trial offering ONPs or other THR products can be proposed in other low- and middle-income countries in the harm reduction area. In addition to diabetes, the proposed study design can be replicated in other clinical settings for smokers with other chronic conditions, e.g., cancer, cardiovascular diseases, and pulmonary diseases, who are susceptible to the detrimental effects of smoking.
To date, no information is available about the impact of oral nicotine pouches on patients with type 2 diabetes who smoke. To our knowledge, DISC will be the first study determining the long- term health impact of using such a THR product in patients with diabetes. In addition, this will be the first intervention in a developing country offering a THR product intervention in a clinical setting for diabetic patients. Diabetic patients need to avoid consuming combustible tobacco products; however, robust evidence-based information is required for governments, policymakers, and clinicians to provide guidance about cigarette substitution. This RCT study design will provide strong evidence to determine the health impact of tobacco harm reduction products among diabetic patients. The length of the study is based on the consideration that changes in the primary endpoint could be reasonably observed as early as three months; however, the extended 12-month follow-up period would firmly establish the long-term impact of the potential of ONPs to reduce risk.
Diabetes mellitus is recognized as a serious public health concern with a considerable impact on human life and is one of the fastest-growing global health emergencies of the 21st century. It is estimated that around half a billion (537 million adults, 20 years-79 years) people had diabetes in 2021; this represents 10.5% of the world’s population in this age group [1]. Type 2 diabetes is the most common diabetes type in adults (~90%). Smoking cigarettes is the leading cause of avoidable mortality, disability, and disease burden [2]. Evidence suggests cigarette consumption has a synergic effect on diabetes and increases the morbidity and mortality of diabetic patients [3]. Exposure to toxic compounds from cigarette smoke increases the risk of vascular damage, endothelial dysfunction, activation of the blood-clotting cascade [4], deleteriously affects lipid metabolism [5], Glycated Hemoglobin A1c (HbA1c) values [6], and eventually the risk of mortality [7-9]. The risk for Coronary Heart Disease (CHD), stroke, and proteinuria is directly related to the number of cigarettes smoked per day [10-12]. Therefore, if reducing exposure to cigarette smoke is urgent for global public health, it is even more so for patients with type 2 diabetes.
The global prevalence of diabetes continues to rise, and there are no signs of it declining or stabilizing. It is more concerning that this burden is increasing rapidly in Low- and Middle-Income Countries (LMICs) as resources are limited in these countries to support the patient group. Almost 80% of diabetes patients live in LMICs, and this is expected to rise to 94% by 2045 [1]. Like prevalence, more than 80% of diabetes deaths occur in LMICs, which makes it the ninth leading cause of mortality in LMICs [13]. Bangladesh is a developing Southeast Asian country with 13.1 million adults living with diabetes in 2021, and this is projected to almost double (22.3 million) by 2045. The International Diabetes Federation (IDF) ranked Bangladesh 8th of the countries with the highest number of diabetic adults and is expected to rise to 7th in 2045 [1]. Bangladesh is not only one of the top ten countries in terms of the number of adults currently living with the disease but is also one of the top ten countries with the highest prevalence of current smokers [14]. Despite the high rate of smoking-related complications, smoking prevalence remains higher among people with diabetes than in the general population [15-18]. Due to inadequate infrastructure for diabetic care, many developing countries are struggling to cope with both the smoking and the diabetes epidemic [19], and Bangladesh is no exception.
The health benefits for people with diabetes who stop smoking begin immediately. Data from type 2 diabetic patients have shown that quitting smoking was associated with a 30% decrease in all- cause mortality, and the benefits for reducing cardiovascular events were more consistent in patients who had stopped smoking for more than 10 years compared to those who had only recently stopped [20]. A descriptive analysis of patients with type 2 diabetes found that the estimated likelihood of CHD risk at 10 years was significantly greater in diabetic smokers compared with former smokers with diabetes [21]. A meta-analysis of men with and without diabetes found that intervention for smoking was the best way to prolong life in patients with diabetes [22]. Current guidance highlights the importance of stopping smoking for patients with diabetes to achieve a better quality of life and to delay the onset and progression of diabetes complications. However, Bangladesh has no dedicated national tobacco cessation service that can provide intervention to either the general population or people with chronic diseases. The increasing prevalence of diabetes with high tobacco consumption poses significant health challenges in Bangladesh. Consequently, one of the important unmet needs for patients with diabetes who continue to smoke in Bangladesh is the delivery of effective smoking cessation interventions. Further studies will be needed to provide clear evidence of which interventions can be valuable for these patients. Consequently, the need for novel and more efficient approaches is required.
Nicotine replacement increases the likelihood of success in smoking cessation. Nicotine is available in different formulations; nonetheless, nicotine-based treatment doubles the chances of success in quitting smoking, regardless of the specific formulation [23-25]. Nicotine Replacement Therapy (NRT) is a medically approved way to treat people with tobacco use disorder; however, the medication is not marketed in Bangladesh [14]. Although not formally regulated as a pharmaceutical product, in the past decade, a strategy of harm reduction for smokers and combustion- free technologies for alternative nicotine delivery products such as electronic cigarettes (e-cigarettes), tobacco heating products, or oral nicotine pouches have been developed. It has been shown that smokers who switch completely to these products instead of continuing to smoke could reduce their relative risks of smoking- related death and disease [26-28]. Identifying an effective and novel intervention for diabetic patients in Bangladesh is challenging. The government of Bangladesh announced that the country’s tobacco control law would be updated with an outright ban on the sales of e-cigarettes [29]. Moreover, e-cigarettes and heated tobacco products require electricity to charge, refill, and clean, which may be impractical for consumers in LMICs, including Bangladesh. Therefore, offering e-cigarettes or heated tobacco products as a form of tobacco harm reduction will not be a suitable solution for smokers in Bangladesh. A novel category of modern Oral Nicotine Pouches (ONPs) has recently emerged. Evidence supports the potential role of ONPs in reducing tobacco harm and helping smokers reduce or stop using combustible cigarettes [30,31]. In addition, oral tobacco use in Bangladesh is common, ranging from unprocessed to processed or manufactured products, including Sada Pata, Zarda, Gul, and Khoinee; over 27% of Bangladeshi adults use smokeless-oral tobacco in one form or another [32]. Nicotine pouches are relatively low-tech and easy to use, have a low environmental impact, with no batteries or other electronic components, and minimal packaging. As their use does not impact bystanders, people can also use nicotine pouches in smoke-free environments where combustible and vaping products may be banned. Therefore, ONPs may have a positive appeal and be a socially acceptable intervention in Bangladesh to reduce smoking- related harm. To date, there is no evidence on how effective ONPs are for smoking cessation among people with pre-existing chronic health conditions, like diabetes.
Given the importance of alternative nicotine products in providing smokers with a satisfactory alternative to cigarette smoking, this clinical research trial will assess the overall effect of smoking cessation intervention among current smokers with type 2 diabetes following the use of ONPs.
Objectives
Primary objectives:
• To assess the efficacy of smoking cessation intervention using Oral Nicotine Pouches (ONPs) compared with usual diabetic care in patients with type 2 diabetes who currently smoke cigarettes/bidis.
• To establish the long-term impact on health outcomes from smoking cessation using ONPs compared with usual diabetic care for smokers with type 2 diabetes.
Secondary objectives:
• To compare the clinical and metabolic consequences in type 2 diabetic patients who successfully quit smoking using ONPs or in usual diabetic care and those who continue to smoke.
• To assess the impact of the intervention (ONPs) on smoking behaviour over the first three months of intervention and at follow-ups assessment at Month-6, Month-9, and Month-12 after the interventional product (ONPs) has stopped being supplied to participants.
• To assess the side effects of the intervention (ONPs) among participants in the intervention group.
• To assess the use and acceptability of the intervention (ONPs) among participants in the intervention group.
• To characterize reasons for interest in using ONPs, reasons for continuing to use ONPs, and reasons for discontinuing to use ONPs.
• To describe the changes in using other tobacco and nicotine products and smoking cessation products and methods during the intervention, phase-out, and follow-up periods.
• To document the suitability of intervention implementation and sustainability of the intervention.
Trial design
Diabetes and Smoking Cessation (DISC) is a prospective two-arm parallel randomized controlled clinical trial designed to assess a smoking cessation intervention with ONPs among current smokers with type 2 diabetes.
Participants will be individually randomized to usual diabetic care (Control) or ONPs free of cost (Intervention). The duration of the active intervention will be 12 weeks, and participants will be followed up in the tapering phase for one month and the non- intervention phase for an additional 39 weeks up to 12 months to assess the long-term effect of the intervention. There is no official medical definition of the long-term effects of medicine or intervention. Evidence suggests abstinence at 12-month follow- up is a good predictor for long-term abstinence and is considered clinically significant [33].
The study will be carried out in line with the Consolidated Standards of Reporting Trials (CONSORT) guidelines and will be reported following the requirements of the CONSORT statement (Figure 1).
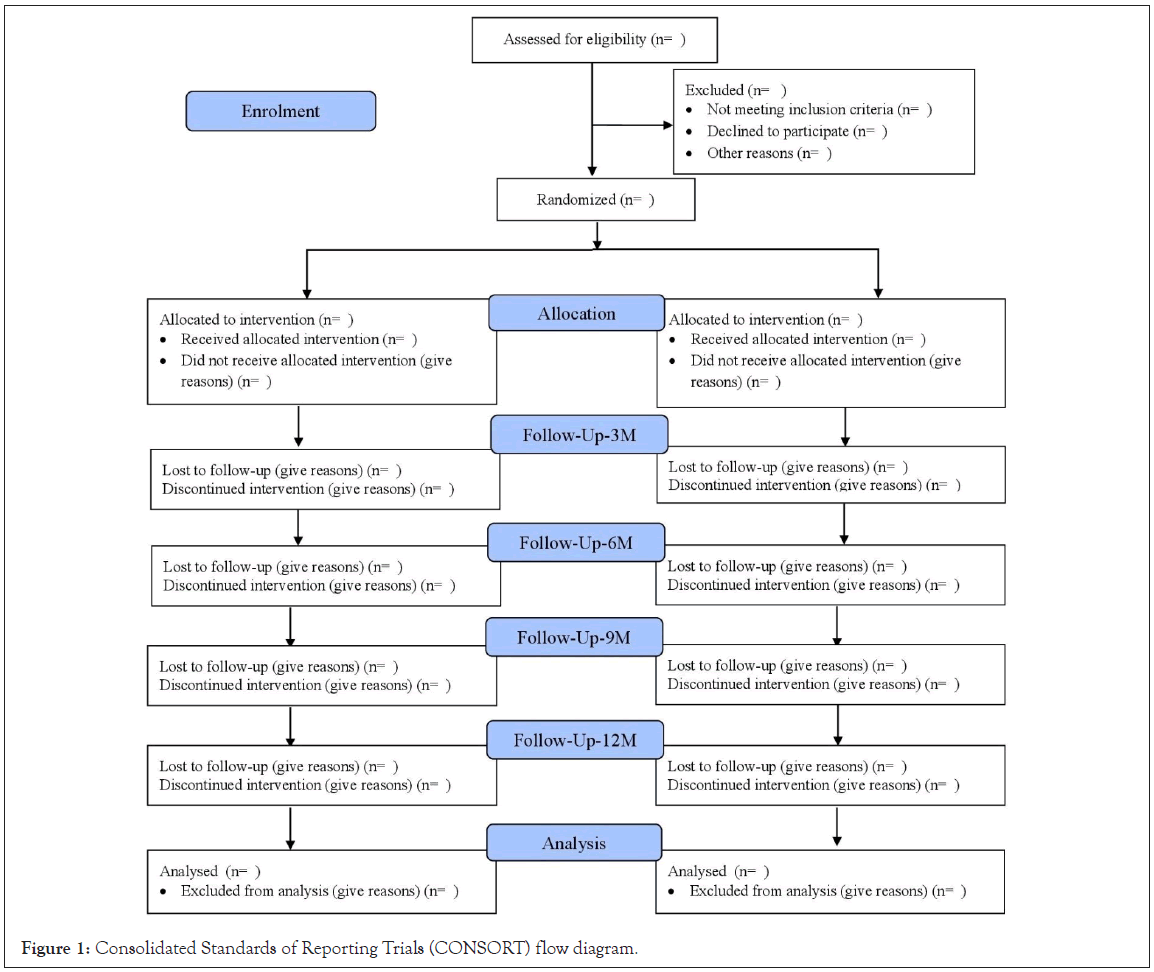
Figure 1: Consolidated Standards of Reporting Trials (CONSORT) flow diagram.
The importance of Patient and Public Involvement (PPI) in health research has been recognized and advocated [38]. It involves patients, ordinary people, and local communities in planning, commissioning, delivering, and evaluating the health and social care services they receive. PPI is considered particularly valuable in clinical trials [39], especially in shaping trial design, selecting outcomes relevant to patients, offering appropriate incentives, and improving recruitment [40].
Therefore, a PPI took place at EMCH on 29 March 2023, which was intended to define the awareness, motivations, and specific needs of diabetic smokers using an interactive workshop. A total of 23 participants, including diabetic patients who are smokers and their caregivers, smoker patients with other illnesses, and interested members of the public in the research project on diabetic patients, were invited to the PPI workshop. The recruitment strategy has been revised based on their comments and preferences (e.g., compensation, desire for a motivational approach, and support for adverse effects). The research team will present the participants with the main results in the future.
Participants may prematurely discontinue the study under the following conditions:
• A participant withdraws his/her consent to participate.
• A participant spontaneously reports a change in characteristics such that he/she no longer satisfies all criteria required for participation (e.g., diagnosed with severe health or mental condition, relocated outside the study area, female participants become pregnant).
• A participant repeatedly refuses or is unable to comply with study procedures and participation requirements stated and agreed to by the participant in the ICFs.
• A participant reports a side-effect that, in the judgment of the PI (Principal Investigator), warrants the participant’s immediate discontinuation of the use of ONPs. The participant would not be provided with any substitute product.
• The study sponsor or implementing organisations terminates the study prematurely.
Reasons for a participant’s premature study withdrawal will be recorded by study staff. Withdrawn participants will not be permitted to re-join the study under any circumstance. The data provided by withdrawn participants up to the point of withdrawal will be retained for analysis.
Delivery of the ONPs intervention will be through home visits. On the 8th day of the study (the day of enrollment will be counted as day 1), the first home visit will be initiated.
Intervention group
Within a week of the baseline measurements, the FRS will visit the participants randomized to the intervention group at their homes to describe and deliver the intervention. At the first visit, each participant will be given two flavors to taste and asked to select their preferred flavor. They will be permitted to use both flavors and one of them or switch between flavors throughout the intervention period. The participants will be trained and counseled on how to use it. Participants will be shown an informational instructional video about pouches on a tablet device. The video will present written and spoken information about pouches in the Bangla language in a manner designed to be understandable to participants of all educational backgrounds using minimal scientific terminology.
FRS will provide a week of supply of ONPs to the intervention participants. In each weekly home visit, participants will be supplied with the appropriate number of pouches (10 per day, 70 for one week) with their preferred flavor to use throughout the week. FRS will collect the used and unused pouches from the previous week. The tapering dose pouches will be delivered weekly following the same procedure for the last 4 weeks. A CAPI questionnaire will be completed during each home visit for delivering the intervention. Participants will be visited weekly for the first 6 months and monthly for the rest of the study period. The intervention participants will be encouraged to use supplied ONPs instead of smoking tobacco cigarettes/bidi for 12 weeks. However, smoking cigarettes/bidi will not result in exclusion from the study.
Control group
The participants randomized to the control will be in exciting diabetes care practice, which is defined as those who are entitled to receive advice about smoking cessation from their local health service. The FRS will visit the control participants each week during the first 6 months and monthly during the last 6 months period, the same as the intervention participants. A short CAPI questionnaire will be completed during each visit.
It is not possible to discuss a smoking cessation intervention with control participants throughout the study, after which they are allocated to the study's control group. However, knowing that the control participants are engaging in harmful behavior, it is considered unethical not to provide any intervention. Therefore, the control participants, who are still smokers, will receive smoking cessation counseling at the end of the follow-up period, Month-12. One-on-one smoking cessation counseling information will typically include the following components: A review of a participant's smoking history and motivation to quit, help identify high-risk situations, and generate problem-solving strategies to deal with such problems. During the counseling, the RP will aim to advise each smoker to stop smoking. Advice will be focused on learning how to reduce a considerable number of cigarettes, discovering why they want to stop and the reasons they have failed before, understanding why they smoke, and how to sustain abstinence. Along with one-to-one counseling, participants will receive a one-page leaflet to support smoking cessation. The leaflet briefly introduces the complications of smoking, tips to make smoking cessation successful, and benefits of quitting smoking.
Following the baseline assessment, participants will be randomly assigned to either the intervention (ONPs users) or control (usual diabetic care) group in a 1:1 ratio by using a block randomization approach with computer-generated random numbers, which offers an equal probability that they will be randomized to the intervention or control group. Using a block size of four, there will be six sequence permutations to which participants will be allocated either to the intervention (A) or control (B) groups: ABAB, BBAA, BAAB, BABA, AABB, and ABBA. One of the six permutations will be selected randomly, and then four participants will be assigned accordingly. The process will be repeated to allocate all participants to either the intervention or control groups for the required sample size. An independent researcher will construct the allocation sequence and seal these in individual opaque envelopes with study identification numbers on the front. Randomization will take place at an individual level after the baseline measurements have been taken and approximately one week prior to the start of the intervention. Therefore, the researcher/field staff will not know the allocation of the participants until after the baseline data collection has been completed. Due to the nature of the study, blinding is not possible; the researcher and participants will be aware that they will be assigned to one of the two groups. However, they will be aware that they have an equal chance of being allocated to the intervention or control group. After randomization, participants will know which group they would be allocated to.
Trained and experienced Field Research Staff (FRS) will collect baseline data through a CAPI questionnaire and complete physical measurements and comorbidities form. Experienced Medical Technologists will collect and preserve the baseline biological samples. The research team conducting such assessments will be blind to group allocation, which will occur subsequently. Following collection of baseline data, participants will be given a 7-days daily consumption diary to record the number of cigarettes smoked per day for the proceeding 7-days. An appointment will be made to visit them, at home, after 7 days.
At the end of the 3-month intervention phase, participants will be invited to attend a hospital visit to complete a 3-month questionnaire and undergo a range of physical assessments and a fasting blood collection and collect comorbidity information. Data on cigarette abstinence, comorbidities, medication changes, and quality of life will be collected from both intervention and control participants. In addition, changes in physical function and disease conditions in relation to pouches use, liking of pouches and intention to use pouches in the future, and reasons for using or stopping pouches will be collected from the intervention participants. Participants will be again invited to attend three further hospital visits to complete a 6-month, 9-months, and 12-month questionnaire and undergo a procedure the same as the Month-3 visit.
The primary screening will be at the Diabetic and Endocrinology departmental clinics; however, other clinics where diabetic patients are more likely to visit will be included as screening clinics to ensure more participation, especially the Internal Medicine department, Rheumatology department, Neurology department, Nephrology department, Ophthalmology department and Cardiology department of EMCH.
Potential participants will be screened for eligibility before being invited to participate. The front desk assistant will initially check patients' records to identify potentially eligible participants and the patient’s consultant will confirm their eligibility. Potential participants will receive a written Informed Consent Form (ICF) with informational sheet, and will be requested to read and, if they are willing to participate, sign a consent form. The study objectives, hypotheses, design, and data collection procedures will be discussed with participants who meet the initial inclusion criteria. The Research Physician (RP) will answer all questions related to the intervention to confirm that each participant has competency, confidence, and sufficient knowledge to use the nicotine pouches safely. The research team will ensure that participants are fully aware of all health warnings associated with pouches and the duration of their involvement in the study. If the respondent expresses an interest in participating and signs the consent form, an interviewer will administer a 10-minute Computer Assisted Personal Interview (CAPI) screening questionnaire to determine the person’s eligibility to participate based on all study inclusion/ exclusion criteria. A Carbon Monoxide (CO) reading will also be taken during this interview, and an exhaled reading CO ≥ 10 ppm is required for inclusion in the study. Following the screening, an appointment date will be arranged to collect baseline data from the qualified participants within the next seven days at the hospital (EMCH).
Subjects responding to the advertisement or referred by the research participants will be contacted by the RP to check the screening criteria at a visit to each participant’s home. All respondents who meet the initial inclusion criteria will be approached, and a participant ICF will be provided. Written informed consent will be obtained after allowing respondents to discuss the requirements for the study and ask any questions. If the respondent agrees to participate, the same CAPI screening questionnaire and CO test will be completed, and a hospital appointment will be arranged for the baseline assessment for those who meet the eligibility criteria.
Participants will be followed up for 12 months in total. Assessment dates will be scheduled to include Month-3, Month-6, Month-9, and Month-12 follow-up visits. The study will consist of the following visits:
• 6 hospitals visits: A screening visit (V1), a baseline visit (V2), one end-of-intervention visit at Month-3 (V3), two follow-up visits at Month-6 and Month-9 (V4, V5), and one final follow- up visit at Month-12 (V6).
• 25 weekly home visits: 12 home visits during the intervention period, 4 home visits during a phase-out period, and 8 home visits during the follow-up period between Month-5 and Month-6 followed by an additional home visit at Month-12.
• 5 monthly home visits between Month-7 and Month-11 of the follow-up period.
Participants from both intervention and control groups will be visited at the same frequency. The overall study procedure is summarized in Figure 2.
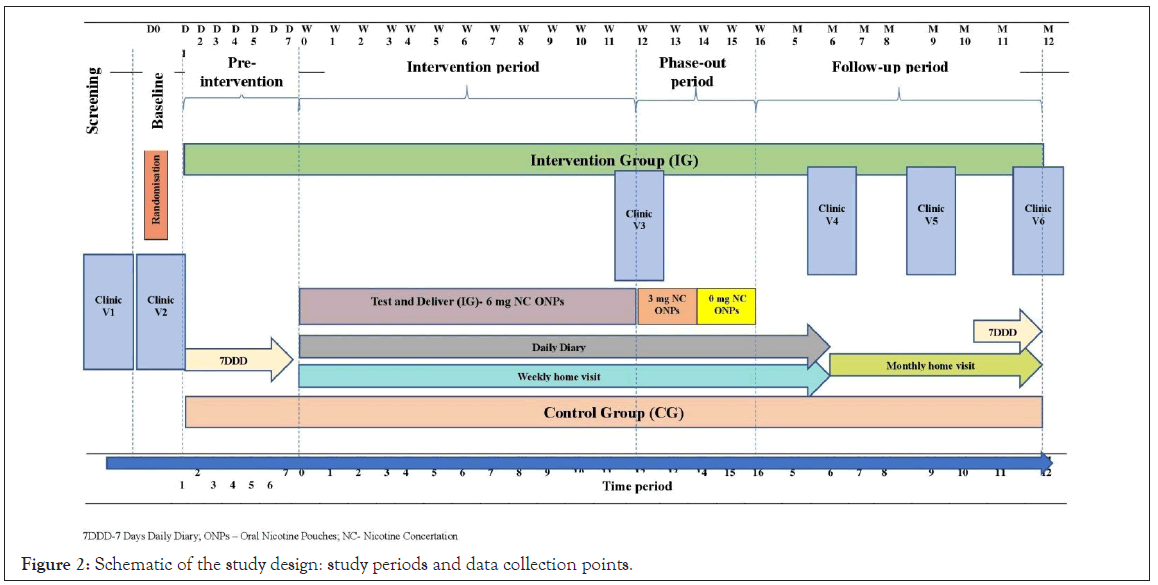
Figure 2: Schematic of the study design: study periods and data collection points.
Sample size
Sample size calculation should be based on attaining sufficient precision for the measures associated with primary and secondary objectives following evidence. One of the primary objectives of this study is to determine the proportion of participants who have achieved point prevalence abstinence by using ONPs at the end of 12 weeks of the intervention phase. The sample size required for this study is calculated based on achieving sufficient precision to analyze this objective. To our knowledge, this is the first proposed clinical intervention trial to assess the impact of switching to a potentially lower-harm nicotine-containing product (ONPs) on smoking in type 2 diabetic patients. No trial with ONPs is available for any participants with chronic disease. It is uncertain whether data from an actual use study of ONPs with general smokers over a different duration of time, e.g., 6-week follow-up (27% switched rate) [31], will have sufficient power to detect statistically significant differences in abstinence between the intervention and control group.
Given the novelty of a clinical study in Bangladesh for a new Tobacco Harm Reduction (THR) product, a more conservative rate is used to ensure sufficient power in the sample size. Therefore, the sample size calculation has been proposed using data from a small pilot study that examined the acceptability of snus and a nicotine pouch amongst hospitalized smokers who have previously tried and failed to quit with NRT (16.6% abstinence in 12 weeks) [35]. Smoking cessation rates are around 5%–20% without interventions [36]. On the assumption that smoking abstinence in the control group will be 5% and 16% in the intervention group at Month-3, a total of 440 participants (220 in each group) is sufficient to detect a more than 10% difference in smoking abstinence between the intervention and control groups at Month-3 with a 20% attrition rate (power 95% and significance level 0.05); 10% difference in smoking abstinence between the intervention and control groups would be considered as clinically significant [37].
Recruitment
Participants will be recruited using three methods. The primary method will be to recruit patients through EMCH consultants’ referrals. Consultants will refer regular and new patients who receive/use the treatments/service facilities at EMCH and are identified as type 2 diabetic smokers. In addition to the primary recruitment method, two other methods will be applied-1) The research team will advertise the study by posting leaflets on the hospital notice board, reception desk, and door of the patient waiting area, and 2) Research participants will be asked to refer potential participants to the study recruitment team (Snowballing).
Data collection and management
All data collection instruments in this study will be programmed into the KoboToolbox. Through an exhaustive iterative process of testing, revision, and correction involving the study investigators and programmers, the offline-to-online programming of all data collection instruments will be thoroughly checked for reliability to the content, structure, format, and operating instructions specified in each instrument.
Data will be managed for monitoring and auditing purposes. A comprehensive and accurate electronic and physical filing system of all study documents will be maintained. Data will be available for inspection at any time and will enable a determination of whether the study procedures are conducted and whether data are stored as described in this protocol and in compliance with any applicable ethical requirements.
Study setting
The proposed RCT will be conducted in Savar Upazila under the Dhaka district in Bangladesh, approximately 24 km from the capital, Dhaka. The study site, Enam Medical College and Hospital (EMCH), is a 1000-bedded private medical college and hospital with outdoor and indoor facilities, established in 2003.
Study population and eligibility criteria
Adult (Age ≥ 18 years) participants with confirmed type 2 diabetes who are current smokers of combustible cigarettes or bidis will be enrolled from the EMCH. Type 2 diabetes will be diagnosed following the criteria of the American Diabetes Association (ADA) [34], and World Health Organization (WHO) [14], criteria will define current smokers. The inclusion and exclusion criteria are summarized in Table 1.
| Inclusion criteria | Exclusion criteria |
|---|---|
| Men or women aged 18 years and over. | Pregnant or breastfeeding or intention to become pregnant during the study. |
| Confirmed type 2 diabetic patients by registered physicians (as defined by ADA). | History of periodontal disease, gum disease or bleeding gums, open mouth sores/ulcers, |
| Smoking at the time of the survey, including Daily and non-Daily smoking (as defined by WHO). | Having received a diagnosis from a doctor of clinically significant cardiovascular, renal, hepatic, neurological, gastrointestinal, respiratory, metabolic, endocrine, haematological diseases or cancer (active malignancy or other condition limiting life expectancy to <12 months) that, in the opinion of the investigator or their appropriately qualified designee, would jeopardize the safety of the participant or impact on the validity of the study results. |
| Smokers of at least 10 cigarettes/bidis per day (maximum of 30 cigarettes/bidis per day) | Having received a diagnosis from a doctor of psychotic disorders, e.g., anxiety, depression, bipolar, Obsessive-Compulsive Disorder (OCD), stress, psychosis, schizophrenia that, in the opinion of the investigator or their appropriately qualified designee, would jeopardize the safety of the participant or impact on the validity of the study results. |
| Smoking for at least the past 12 months regularly. | |
| Exhaled carbon monoxide level of at least 10 ppm. | |
| Willing to try using ONPs instead of smoking cigarettes/bidi for 3 months. | |
| Permanently living in the study area and have no plan to move in the next 12 months. | Self-rated physical or mental health as “poor”. |
| Able to provide fully informed consent to take part in the research study. | Currently participating in another consumer or medical or smoking cessation study. |
| Willing and able to provide home address and contact information. | Employed by, in litigation with, or has a financial interest in a tobacco or nicotine product company. |
| Willing and able to comply with study data collection requirements, including attending clinic visits, completing Daily diaries, and attending the interviews at home. | Close friends with, or relative to, individuals employed by, in litigation with, or has a financial interest in a tobacco or nicotine product company. |
Table 1: Inclusion and exclusion criteria.
Interventions
The intervention will be 6 mg dry nicotine pouches in two flavors- Mint and Neutral. Participants will be instructed to use a maximum of 10 pouches per day. The supply of 6 mg nicotine pouches will continue for 12 weeks and will not be stopped abruptly. Instead, a gradual decrease in nicotine strength will be introduced at the end of the intervention. Participants of this trial will be supplied with ONPs with nicotine strength varying according to the following schedule: 6 mg for 12 weeks, 3 mg for 2 weeks, and 0 mg for 2 weeks.
Outcomes
Outcome measures will be guided by the study objectives and listed in Table 2.
| Objective | Outcomes | Measure | Instrument |
|---|---|---|---|
| 1 | Point prevalence abstinence | Proportion of participants who self-report consumption of no smoking in the past 7-days during Week-12 and have an expired carbon monoxide reading of ≤10 ppm at Month-3 hospital visits | Daily diary CO test |
| >=50% reduction in average CPD | Proportion of participants who self-report>50% reduction in average CPD between Week-12 compared to the average CPD reported at baseline (Week-0) | Daily diary | |
| Average CPD | Total number of cigarettes smoked in past 7 days during Week-12 | Daily diary | |
| 2 | Clinical and metabolic outcomes related to diabetes | Mean/Median/percentages of Pulmonary function, Liver function, Inflammatory biomarker, Body weight, Body Mass Index, Blood glucose, Blood pressure, Lipid profile, HbAc1 at Month-3 and Month-12 | Clinical measures |
| Health outcomes | Proportion of participants reported new diagnoses of diseases, changes in disease condition, changes in physiological function and medication at Month-3 and Month-12. | End-intervention questionnaire Follow-up questionnaire | |
| Quality of life | Mean score of Short Form (SF-12) quality of life measure at Month-3 and Month-12 | End-intervention questionnaire Follow-up questionnaire | |
| 3 | Clinical and metabolic outcomes | Mean/Median/percentages of Pulmonary function, Liver function, Inflammatory biomarker, Body weight, Body Mass Index, Blood glucose, Blood pressure, Lipid profile, HbAc1 at 3-, 6-, 9- and 12 months | Clinical measures |
| 4 | Point prevalence abstinence | Proportion of participants who self-report consumption of no smoking in the past 7-days and have an expired carbon monoxide reading of ≤10 ppm at 3-, 6-, and 12 months | Daily diary CO test |
| Continuous abstinence | Proportion of participants who self-report consumption of no smoking since the introduction of intervention and have an expired carbon monoxide reading of ≤10 ppm at 3-, 6-, and 12 months | Daily diary CO test | |
| Self-reported point prevalence abstinence | Proportion of participants who self-report consumption of no smoking in the past 7-days at 3-, 6-, and 12 months | Daily diary | |
| Self-reported continuous abstinence | Proportion of participants who self-report consumption of no smoking since the introduction of intervention and have an expired carbon monoxide reading of ≤10 ppm at 3-, 6-, and 12 months | Daily diary | |
| Average CPD | Total number of cigarettes smoked in past 7 days at 3-, 6- and 12 months | Daily diary | |
| >=50% reduction in average CPD | Proportion of participants who self-report >50% reduction in average CPD at Week-12, Week-24, and Week-52, compared to the average CPD reported at baseline (Week-0) | Daily diary | |
| 1%-49% reduction in average CPD | Proportion of participants who self-report 1-49% reduction in average CPD at Week-12, Week-24, and Week-52, compared to the average CPD reported at baseline (Week-0) | Daily diary | |
| No change in average CPD | Proportion of participants who self-report no change in average CPD at Week-12, Week-24, and Week-52, compared to the average CPD reported at baseline (Week-0) | Daily diary | |
| Increase in average CPD | Proportion of participants who self-report increased in average CPD at Week-12, Week-24, and Week-52, compared to the average CPD reported at baseline (Week-0) | Daily diary | |
| 5 | Side-effects from the use of ONPs | Proportion of participants who endorse side-effects for current use of ONPs at Month-3 and Month-4 | Weekly questionnaire |
| 6 | Use of ONPs | Number of ONPs consumption days in the past 7 days during the intervention and phase-out period | Daily diary |
| Average ONPs | Average ONPs in the past 7 days during the intervention and phase-out period | Daily diary | |
| Regular use of ONPs | Proportion of participants who consumed 31 ONPs on 3 3 days per week for 3 3 weeks during the intervention period and phase-out period | Daily diary | |
| Liked the intervention | Proportion of participants who reported a liking score>5 on the product liking question at Month-3 | End-intervention questionnaire | |
| Intention to use intervention again | Proportion of participants who reported an intent to use the product again with a score>5 on the intention to use question at Month-3 | End-intervention questionnaire | |
| 7 | Reasons for use and stopping use of ONPs | Proportion of participants who endorse each reason for: a. Interest in using ONPs at baseline b. Continuing to use ONPS at Month-3 c. Stopping the use of ONPS at Month-3 | Product select questionnaire. End-intervention questionnaire |
| 8 | Use of other tobacco, nicotine, and stop smoking products | Proportion of participants who have used each of a list of tobacco products, nicotine products, and stop smoking products in the following timeframes: a. Ever used. b. Past 30 days. c. Past 7 days | Baseline questionnaire |
| Proportion of participants who have used each of a list of tobacco products, nicotine products, and stop smoking products in the past 7 days | Weekly questionnaire | ||
| Proportion of participants who have used each of a list of tobacco products, nicotine products, and stop smoking products in the following timeframes: a. Ever used. b. Past 30 days. c. Past 7 days | Monthly questionnaire Follow-up questionnaire | ||
| 9 | Suitability of intervention | a. Participants experience b. Research staff experience | Qualitative interview |
Note: Abbreviations: CPD = Cigarettes Smoked Per Day, ONPs= Oral Nicotine Pouches. CO=Carbon Monoxide
Table 2: Outcomes, measures, and analysis plan used in the analysis of study objectives.
Citation: Haseen F, Hossain AS, Rahman N, Chowdhury AM, Rana S, Hossain T, et al. (2023) A Randomized Controlled Trial to Assess the Long-Term Health Effects of Smoking Cessation Intervention through a Tobacco Harm Reduction Product among Type 2 Diabetic Smokers in Bangladesh- Study Protocol of the Disc Trial. J Clin Trials. 13:539.
Received: 18-Aug-2023, Manuscript No. JCTR-23-26171; Editor assigned: 21-Aug-2023, Pre QC No. JCTR-23-26171(PQ); Reviewed: 04-Sep-2023, QC No. JCTR-23-26171; Revised: 11-Sep-2023, Manuscript No. JCTR-23-26171(R); Published: 18-Sep-2023 , DOI: 10.35248/2167-0870.23.13.539
Copyright: © 2023 Haseen F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.