Journal of Clinical & Experimental Dermatology Research
Open Access
ISSN: 2155-9554
ISSN: 2155-9554
Research Article - (2021)Volume 12, Issue 1
Introduction: Age related changes in hair and skin impact quality of life. Interventions to mitigate these changes are of interest.
Aim: To examine the safety and efficacy of LustrivaTM (a novel source of biotin and silicon) at a high or lower dose compared to placebo for impacts on hair and skin.
Materials and Methods: In a randomized, double-blind study, 90 healthy female subjects with self-reported thinning hair who met Savin/Ludwig Scale criteria I-2 to II-1 by physician evaluation were randomized to one of three groups for 12 weeks (n=30/group): LustrivaTM High-Dose (LHD), LustrivaTM Low-Dose (LLD) or Placebo (PL). Hair quality and thickness measured by the TrichoScan HD testing system and skin parameters (facial wrinkles, fine lines, skin texture, skin color evenness, skin elasticity) measured by the Antera 3DTM System and the CutometerTM Dual MPA 580 system.
Results: There was a significant increase in hair thickness measured by change in % vellus hair and % terminal hair and in the ratio of % vellus to terminal hair in LHD compared to PL at Week 3, maintained throughout the study (p=0.029). LHD had a significant decrease in facial wrinkles (12 Weeks) measured by a change in maximal wrinkle depth vs. PL (p=0.031). After 12 weeks compared to baseline LHD significantly improved facial wrinkle Maximum Depth, Indentation Index and Score, facial fine lines Indentation Index and Score, and facial texture Maximum Height, Roughness and Score (p<0.05), no change in PL. There were no changes for skin elasticity between groups. For some hair and skin parameters, LLD showed improvements less than LHD but that approached significance (p<0.1). All groups improved in subjective nail endpoints vs. baseline with no significant differences between groups. No adverse events reported.
Conclusion: LHD significantly increased hair thickness and reduced facial wrinkle depth compared to placebo and performed better than the LLD in most parameters. Future studies are warranted.
Dermatology; Hair; Silicon; Biotin
Hair loss, hair thinning, skin wrinkles, and nail brittleness are common dermatological complaints, with some patients reporting that these changes negatively impact their quality of life [1]. Hair loss can be acute or chronic and affects at least 50% of women by age 50 [2]. Generally, adult women shed 50 to 100 single hairs per day. While hair shedding is part of the natural hair cycle, noticeable hair loss is not. It is estimated that more than 50% of women will experience noticeable hair loss. In fact, female-pattern hair loss affects at least 30 million women in the United States [3]. It is estimated that the business market size for hair loss products (pharmaceutical and non-pharmaceutical) is $4 Billion USD, with the average women spending upwards of $55,000 USD or more in their lifetime on haircare products [4-6]. The data also reveal that the health and appearance of nails is important to women. Market analysis has determined that women spend $8.36 billion a year on services offered in nail salons [7]. Amongst the reasons products are purchased in this category are that skin exposure to ultraviolet light over time may lead to photoaging of the skin and wrinkling of the skin. It is interesting to also note that on average, a “Gen-X” women uses on average six different skin beauty products daily with an average monthly spend of $42 (˜$504/year) [6,8]. Over a lifetime, one recent survey found that women will spend $225,360 on products that are intended to improve physical appearance [6]. More than 25 percent of the dollars spent by women on appearance is directed at facial products (lifetime spend; $51,120) [6]. Thus, from a financial spend vantage, it is obvious that the appearance of hair and skin, as well as nails, are of importance.
It is known that the appearance of hair, skin and nails changes throughout the lifecycle. These changes are due to genetics, environment, structural changes, and aging, itself [9]. Hair loss can have a significant psychological impact resulting in symptoms of depression [10] and diminished quality of life, especially in women [11,12]. Women and men of all ages can be affected; however, the thinning presents differently in women in that it is diffuse [13]. Therefore, interventions to prevent and or mitigate these conditions are of great interest. Several nutritional interventions have been suggested including eating a healthy, well-balanced diet that contains adequate daily intake of vitamins and minerals, especially trace minerals. Several studies utilizing various vitamin and mineral supplements to improve brittle nails have been conducted with mixed results [14,15]. In addition, there has been increased attention in the literature for non-invasive strategies for supporting healthy skin and for offsetting some of the signs associated with photoaging. This has led to research supporting the role of a healthy, nutrient-rich diet for promoting skin health. However, exactly what this means and exactly how individual dietary components contribute is less known [16]. In addition to a healthy diet, dietary supplements are thought of as a potential intervention for the appearance of hair, skin or nails. The watersoluble vitamin Biotin has been shown to play a major role in the synthesis of protein including keratin, the fibrous protein that forms the main structural constituent of hair and nails. It is also a coenzyme for the mitochondrial carboxylases in hair roots. By supporting the processes of formation of keratin and the differentiation of epidermal cells in hair and nails, Biotin can help improve their condition [17,18]. Studies have shown that Biotin supplementation improves nail thickness and splitting. Silicon is the third most abundant trace mineral in the human body and is important for the synthesis of collagen, activating hydroxylation enzymes, and improving skin strength and elasticity [19]. It has also been shown to improve hair brightness and to prevent hair loss [20]. Because of the potential for Biotin and Silicon to improve common hair, skin and nail conditions and thus improve quality of life in many consumers, this study aimed to examine the safety and efficacy of LustrivaTM (a novel compounded dietary ingredient) at a high or lower dose as compared to placebo for impacts on hair and skin in otherwise healthy women.
This study included 90 randomized adult females (21-65 y.o.) who met the Savin/Ludwig Scale Criteria I-2 to II-1 by physician evaluation for female hair loss and pattern for inclusion [21-23]. This scale for staging the potential study participant was utilized because it measures the density and the thickness of the hair, allowing for classification of the stages of hair loss or change. Additionally, the study participants verbalized that they were not happy with the appearance of their hair (self-professed thinning) or skin (i.e., signs of photodamaged skin) and were interested in learning if a compound dietary supplement would make any differences in their hair or skin appearance. Subjects had to meet a strict inclusion and exclusion criteria for enrollment.
Study inclusion criteria included
• Adult females aged 21 to 65 years of age inclusively at the time of screening, who were not happy with the appearance of either their hair or skin (i.e., signs of damaged hair or photodamaged skin) but were otherwise healthy and stated that they had selfprofessed thinning of the hair.
• The Principal Investigator (PI) confirmed that subject qualified for the study by meeting the Savin/Ludwig Scale Criteria I-2 to II-1 for entry.
• In good health and assessed as eligible by screening blood pressure, screening heart rate, screening blood test for metabolic panel, CBC with differential, physical exam, and by medical plus surgical history per the PI.
• Agreed to maintain a stable lifestyle with no change in exercise or diet for the duration of the study.
• Agreed to maintain a consistent shampooing frequency, same shampoo and conditioner (as applicable), and cut and color of their hair for the duration of the study, as long as the cut/color did not include the area of TrichoScan HD analysis (near the crown of the head).
• Agreed to provide a detailed list of all facial cosmetic products (including night creams, lotions, facewashes, etc.) that they were using daily or frequently and not to change this regimen for the duration of the study.
• Agreed to avoid all tanning (sun or artificial such as tanning beds, sprays and other topical applications) and sun burning for the entire duration of the study and confirmed avoiding them 2 weeks prior to screening. If it happened accidentally, it had to be documented in the source documentation and the CRF.
• Agreed not to utilize any new over the counter, commercial or other products that were marketed and promoted for enhancing aspects of their hair or skin (e.g., ViviscalTM, BioSilTM, NutrafolTM, etc.) for the entire duration of the study and confirmed not using them one month prior to the screening visit.
• Were able to refrain from strenuous exercise/activity for at least 24 hours prior to each visit. Able to refrain from alcohol (or alcohol-containing beverages and foods) consumption for at least 24 hours prior to each visit.
• Agreed to refrain from taking any vitamins or dietary/herbal supplements containing silicon or biotin for at least 7 days prior to the baseline visit and throughout the study, except for the study product.
• Subjects agreed on the visit days to refrain from using lotions, creams, makeup, or other products on their face until after completion of the study visit assessments.
Objective measurements
Skin measurement: The skin appearance of wrinkles, scars, redness, pigmentation, texture and skin color were evaluated on the Antera 3DTM System. Briefly, the Antera 3DTM system is a validated objective system for determining changes in the skin. An Antera 3D evaluation of wrinkles, scars, redness, pigmentation, texture and skin color was completed at baseline and throughout the study period (over a 12-week period, measuring changes from baseline). This diagnostic tool utilizes a technology related to shape from shading, photometric stereo measurements, with the aim to reconstruct the skin in 3D thanks to multiple images taken under different light sources with 7 different wavelengths spanning most of the visible spectrum [24,25]. The software automatically calculates the objective outcome measures.
Skin elasticity was measured by using the CutometerTM Dual MPA 580TM system. Per the manufacturer, the measuring principle of the CutometerTM is based on the suction method, where negative pressure deforms the skin mechanically. The pressure is created in the device and draws the skin into the aperture of the probe and after a defined time, releases it again [26].
Hair analysis: Evaluation of changes in hair was conducted by using the TrichoScan HD system. The TrichoScan HD testing system is a computerized method to determine hair density and the status of hair roots of scalp hair. It calculates responses to treatment in patients (study subjects) [27,28]. In order to conduct the procedure, a small concealed portion of the subject’s hair was clipped with an electric clipper; care was taken that the hair clipped was even and not too short. Next, the optical contact plate that had a LED light-ring to ensure proper lighting was pressed firmly onto the scalp, also, an alcoholic disinfection spray was used to enhance the optical plate. This helped the hair lay flat onto the scalp and ensured that the image was taken at the same distance from scalp. Next, the image was taken by a camera that was completely controlled by software that ensured settings like zoom, contrast, resolution, compression, and others were the same from image to image. Measurements were conducted by a trained professional per the user manual who was aware of the technical requirements like contrast, lightening and ensuring no air bubbles and no hair dye remnants. Hair dyes were used to enhance contrast for white, grey, or fair hairs, if deemed appropriate [29].
Photographic system: A NikonTM D5300 with a TAMRON Model F017 lens was used to obtain photographs of the frontal face, crown, facial profile (left side of the participant), and back of the head. All photographs were taken in the same location with same lighting without using flash or portrait mode. Frontal face and profile photographs were captured from bottom of the chin to top of the head at resting face position without smile. The crown photograph was taken with the natural hair part either down the middle or side from a frontal view, facing the subject. Back of the head photograph (with hair down) was also taken. For the frontal, crown, and profile photographs, all the identifying features (eyes, nose, mouth, ears, birth marks, moles, and scars) were de-identified per standard operating procedures of the clinic (QPS-Missouri, Springfield, MO) and the study Sponsor (JDS Therapeutics, Harrison, NY).
Study products
1. High-dose LustrivaTM: Comprised of 146.5 mg inositol-stabilized Arginine Silicate (ASI) with 11.7 mg magnesium biotinate providing 10 mg silicon and 10 mg of biotin and inactive ingredients: Dicalcium phosphate (Dihydrate), silicon dioxide, magnesium stearate, gelatin and titanium dioxide. Subjects were instructed to consume one capsule daily.
2. Low-dose LustrivaTM: Comprised of 146.5 mg inositol-stabilized arginine silicate (ASI) with 3.5 mg magnesium biotinate providing 10 mg silicon and 3 mg biotin and inactive ingredients: Dicalcium phosphate (Dihydrate), silicon dioxide, magnesium stearate, gelatin and titanium dioxide. Subjects were instructed to consume one capsule daily.
3. Placebo (inactive ingredients: Dicalcium phosphate (Dihydrate), silicon dioxide, magnesium stearate, gelatin and titanium dioxide). Subjects were instructed to consume one capsule daily.
4. The study product and placebo were supplied by JDS Therapeutics, Harrison, NY.
5. The participants and the Investigator/study staff who conducted the study procedures were blinded by packing the study products that had the same appearance into identical DispillTM blister packs.
Statistical methods and approach
As this study was exploratory in nature, the sample size was considered a convenience sample of 90 randomized subjects, given 30 per group (n=30 low-dose LustrivaTM; n=30 high-dose LustrivaTM and n=30 placebo). For analysis, the study employed Full Analysis Set (FAS) for any efficacy evaluation. A Full Analysis Set included all randomized subjects who have received at least one dose of study product and have at least one baseline efficacy assessment and one post-baseline efficacy assessment.
A Per Protocol Analysis is considered a sub-analysis of the FAS dataset. A Per Protocol analysis was also undertaken (analysis of the data for those subjects who started and completed the study with all data being accounted for).
For determination of safety, all subjects who received at least one dose of the study product were included in the safety analysis.
For the continuous dependent variables, paired t-test or Wilcoxon signed rank test was used to determine the significance of change from baseline within each group depending on whether or not the data was normally distributed. The analysis of variance (ANOVA) or Kruskal-Wallis test was applied to compare the change from baseline among the low-dose group, high-dose group, and placebo group depending on normality distribution and variance structures of three groups. The same analysis (ANOVA) was applied to percent change from baseline, together with summary.
For categorical dependent variables, chi-square test or Fisher’s exact test was applied to compare the difference in proportion among the three groups.
In addition, the analysis of covariance (ANCOVA) model was applied to analyze the efficacy endpoint, with the change from baseline as the response variable, treatment, visit, the interaction of treatment and visit as fixed effect, and finally, the baseline score as a covariate. Based on the model, the overall least squares means (LS Means) for low dose group, high-dose group, combined treatment group, and placebo group was calculated, together with 95% confidence interval. The differences in overall LS Means between the low-dose group and placebo group, high-dose group and placebo group, combined non-placebo group and placebo group, and low-dose group and high-dose group, was calculated, together with 95% confidence interval. Furthermore, the similar LS Means and difference in LS Means for different visits was also calculated.
Institutional Approval-This study was executed by the research site and company QPS. Specifically, The QPS-Missouri (Springfield, MO) clinical site executed and conducted this study with the IRB approval by the Bio-Kinetic Clinical Applications on October 28, 2019 and study oversight by Nutrasource (Guelph, Ontario, Canada).
Screening and enrollment
161 subjects were screened for this study. Of the 161 screened 90 subjects were deemed eligible and randomized. Eight-nine (89) subjects out of the 90 enrolled were included in Full Analysis. The Safety study population included all 90 subjects that were randomized. The Per Protocol analysis was carried out on 88 subjects (two subjects in the placebo group did not finish the study, one which was lost-to-follow-up and the second person discontinued due to an unrelated serious adverse event).
Demographics
The 90 subjects enrolled were all female, 46.9 ± 11.69 years of age, and predominately Caucasian (86 of the 90 subjects), with three African Americans and one American Indian participant (90 total). Subjects had a mean height of 165.4 ± 6.00 cm and a body weight of 76.99 ± 12.6 kg. The corresponding body mass index for the overall participants was 28.17 kg/m2.
Skin parameters
Facial skin wrinkles: Both the Lustriva HD and the Lustriva LD groups experienced significant improvement in the Wrinkles Indentation Index from baseline to Week 3 (~day 21) [Lustriva HD=-0.367 (0.1779), p=0.0405; Lustriva LD=-0.417 (0.1779), p=0.0202], while the PL group experienced no change. By the end of the study (day 84), only the Lustriva HD group achieved significant improvement in the maximal wrinkle depth [Lustriva HD=-7.0 (2.9), p=0.0253], whereas the other groups did not experience such a change (p>0.05). Additionally, the results (by t-test) from the Antera 3DTM system tests indicated a significant improvement in maximum depth of wrinkles from baseline to Visit 5 (end of study visit) in Lustriva HD [-8.0 (18.9)], when compared with Placebo [1.0 (12.1)], p=0.031. Lustriva HD over 12-weeks had the greatest impact on facial wrinkle indentation index (Figures 1 and 2).
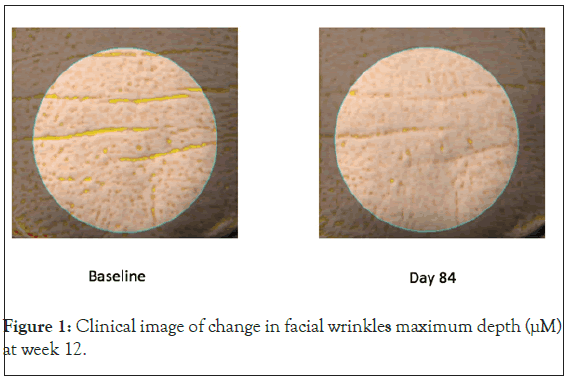
Figure 1: Clinical image of change in facial wrinkles maximum depth (μM) at week 12.
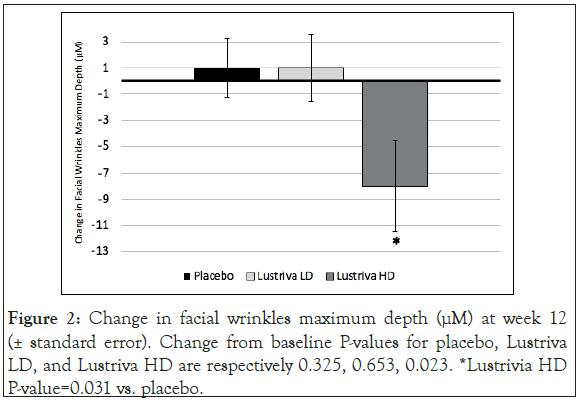
Figure 2: Change in facial wrinkles maximum depth (μM) at week 12 (± standard error). Change from baseline P-values for placebo, Lustriva LD, and Lustriva HD are respectively 0.325, 0.653, 0.023. *Lustrivia HD P-value=0.031 vs. placebo.
Facial fine and overall fine lines: Both the Lustriva HD and Lustriva LD demonstrated statistically significant improvements in the facial fine lines score over the course of the study, whereas the PL group did not [Lustriva HD=-0.216 (0.0854), p=0.0124 and Lustriva LD=-0.222 (0.0853), p=0.0099]. The improvement for the Lustriva HD and the Lustriva LD was also significant for the overall fine lines score [Lustriva HD=-1.473 (0.5766), p=0.0115 and Lustriva LD=-1.412 (0.5756), p=0.0152], which was not significant for the placebo group (p>0.05).
Skin texture: For skin texture (maximum height), the Lustriva HD experienced a significant improvement over the course of the study, [(day 84) Lustriva HD=-0.008 (0.0036), p=0.0239], which was not true for the Lustriva HD or Placebo groups (p>0.05). Additionally, the Lustriva HD group had significant improvement in skin texture roughness by study visit 3 [(day 21), Lustriva HD=-0.504 (0.1894), p=0.0085], which was maintained for the duration of the study [(day 84) Lustriva HD=-0.705 (0.2558), p=0.0065]. Lustriva LD and Placebo did not achieve significant changes for this outcome parameter.
Other skin parameters: For skin color, overall the data for between group differences, over the course of the study, the Lustriva LD was found to be significantly better than the placebo [C=0.075 (0.0326), p=0.0072]. There were no remarkable changes noted for skin elasticity between any of the groups.
Nails: All groups improved vs. baseline in the three subjective nail endpoints (appearance, strength, and growth) measured by Likert scales though there were no significant differences between groups.
Hair parameters: Changes in hair growth, turnover and health were evaluated by the TrichoScan HD. Changes in percent vellus and percent terminal hair (and the ratio of the two) were of study interest. There was a significant change in % vellus hair and % terminal hair ratio as well as in the ratio of % vellus to terminal hair in the Lustriva HD group as compared to Placebo by Week 3 (day 21) and remained significant at Week 12 (p=0.029). Over the course of the study, the Lustriva HD group outperformed the PL group for change in vellus hair [Lustriva HD=-13.18 ± 15.28 vs.-5.73 ± 9.32; p=0.029], there were no differences between the Lustriva LD and PL for this outcome (p>0.05) (Figures 3 and 4).
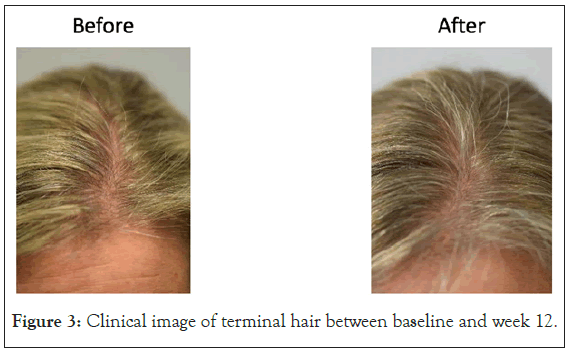
Figure 3: Clinical image of terminal hair between baseline and week 12.
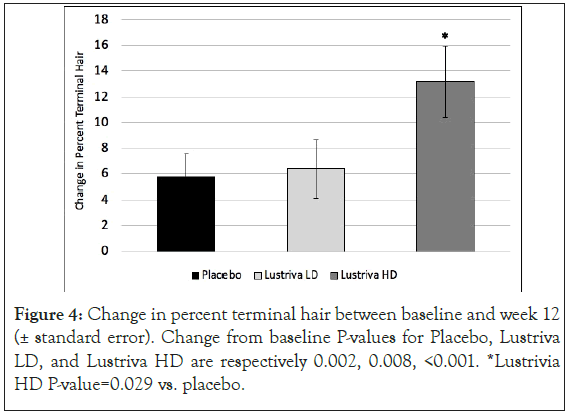
Figure 4: Change in percent terminal hair between baseline and week 12 (± standard error). Change from baseline P-values for Placebo, Lustriva LD, and Lustriva HD are respectively 0.002, 0.008, <0.001. *Lustrivia HD P-value=0.029 vs. placebo.
Safety: This study did have one Serious Adverse Event (SAE). The SAE occurred in the Placebo group and was deemed by the study physician to be unrelated to the study. There were no safety concerns regarding the Lustriva HD or the Lustriva LD or the Placebo groups as all safety monitoring signals remained within clinically normal values (comprehensive metabolic panel, complete blood count with differential, monitoring of blood pressure and related items).
This study evaluated the impacts of two different doses of a commercial compound novel dietary supplement chiefly comprised of inositol-stabilized Arginine Silicate (ASI) and magnesium biotinate. This novel compound dietary supplement provides arginine, silicon, magnesium and biotin to the end user. In pre-clinical testing and evaluation, the tested dietary supplement was able to achieve significant improvement in skin protection (appearance and elasticity) against photoaging (rodent ultraviolet radiation model) as compared to placebo [30]. The product tested in this human trial, was also evaluated in a pre-clinical rodent model for potential efficacy in hair and nail growth. In the pre-clinical study, it was found that the unique dietary supplement promoted significant positive changes in hair growth and hair density, while also supporting nail growth [30]. The two pre-clinical studies yielded signals of follow-up in a human model. In this clinical study, impaired nails were not one of the entry criteria which may explain why all groups saw improvements vs. baseline though there were no significant differences between groups. Arginine by itself is known to be a vasodilator and positive promoter of nitric oxide production. L-arginine is the substrate for the enzyme Nitric Oxide Synthase (NOS), which is responsible for the endothelial production of nitric oxide [29]. Dietary supplementation with L-arginine has been shown to have human health benefits, including significantly improving endothelial function in individuals even without vascular disease (healthy subjects) [31]. Blood flow to the skin is neurally controlled by the adrenergic vasoconstrictor system and an active vasodilator system [32]. The vasodilator pathways mediate downstream nitric oxide dependent vasodilatation, which is needed for the full reflex vasodilatory response in humans, the nutritional driver of NO production is the amino acid arginine [33]. The form of arginine used in this study (inositol stabilized arginine silicate) has been shown to significantly enhance blood flow velocity, which can be secondary to improved vasodilation [34]. Understanding that L-arginine is the common substrate for both Nitric Oxide Synthase (NOS) and arginase is important, as arginase also catalyzes the conversion or arginine to urea and ornithine, which is the precursor to proline. Proline has importance in skin and other tissue integrity, especially for collagen synthesis [35]. Arginine, in addition to being able to be converted into proline, also can be incorporated into collagen itself [36]. The arginine-derived nitric oxide and arginine itself is said to have an important role in the overall health and appearance of skin, though this has not been well-tested in terms of outcomes (i.e., can arginine supplementation have a positive impact on the appearance of skin), hence this study evaluated the question [37].
In this study, there were noticeable significant changes in aspects of facial skin appearance with a significant reduction in facial wrinkles and wrinkle depth, fine lines, skin texture and skin roughness most consistently for the Lustriva HD product. While there were no signs, symptoms or overt concerns regarding any nutrient deficiency or insufficiency in the diets of the study participants, it is wholly possible by the mechanism of action of how Lustriva HD works, that the enhanced blood flow and greater nutrient delivery translated to better appearing skin (by objective measurements).
Within the ingredients of the Lustriva HD and LD product is magnesium biotinate. Magnesium biotinate has been shown to be 40 times more soluble than biotin alone and has been patented as WIPO Number WO2018045244A1 with the US Patent number of 2017049757 W 20170831. A recent pharmacokinetic study demonstrated that this version of biotin is also well absorbed in a dose dependent manner in humans [38]. Biotin plays a role in many metabolic pathways in the body and it is an essential dietary cofactor for mammalian carboxylase enzymes [39]. In short, biotin is a water-soluble vitamin that participates as a cofactor in gluconeogenesis, fatty acid synthesis and branched chain amino acid catabolism. Biotin functions as the carboxyl carrier for biotindependent carboxylases. Its covalent attachment to carboxylases is catalyzed by holocarboxylase synthetase. Biotin deficiency is rare. Biotin as a dietary supplement for normal healthy people without deficiency or any inborn error of metabolism on its own may not be an ergogenic aid for hair health or skin health. However, within the confines of this study, the biotin-containing compound nutritional product did demonstrate benefits over placebo for objective outcomes as related both to facial skin appearance and hair growth. Studies utilizing biotin supplementation alone for hair loss secondary to a laparoscopic sleeve gastrectomy (over a one-year period) provided mixed results with the conclusion indicating biotin on its own has low efficacy to prevent hair loss [40]. Interestingly enough, a recent survey study found that 43.9% of physicians prescribe biotin primarily for hair or nail disorders, which is an indictor and barometer of physician’s standards of care for their patients [41]. The biotin form utilized as part of the study compounded nutritional product (LustrivaTM) is a novel one in that it is magnesium biotinate. This means that magnesium is also being delivered to the body upon ingestion. It is interesting to note that magnesium is one of the mineral elements that as we age, it appears to be in greater concentrations in the hair (meaning excretion of calcium into hair increases as we age past 20 to 25) [42]. The National Health and Nutrition Examination Survey (NHANES) revealed that much of the United States does not consume adequate amounts of magnesium. The median intake of magnesium in the diet was 326 mg/d for Caucasians, 237 mg/d for African Americans and 297 mg/d for Mexican American men. For women, the median intake was 237 mg/d for Caucasians, 177 mg/d for African Americans and 221 mg/d for Mexican Americans. All of these amounts are below the daily recommended allowance for magnesium (males: 400 to 420 mg/d and females: 310 to 320 mg/d for those aged 19-30 and 31 to 51+) [43]. Hence, it can be stated that the nutritional compound product which was evaluated in this study may have ameliorated some of the vitamin or mineral insufficiency in the diets of the study participants.
In conclusion, there were statistically significant between group changes in the Lustriva HD group compared to placebo for facial wrinkles using the Antera 3DTM system and % vellus and % terminal hair, as well as for the ratio of percent vellus to terminal hair, as tested via the TrichoScan HD system. The Lustriva HD group overall appeared to perform better than the Lustriva LD group as compared to Placebo in most of the study parameters. In this study, the test products, Lustriva HD and Lustriva LD, have shown statistical significance over the placebo for hair and skin endpoints with no safety concerns.
Funding for the study provided by JDS Therapeutics, Harrison NY.
The authors are employees of Nutrasource, the CRO that oversaw the study.
Citation: Kalman DS, Hewlings SJ (2021) A Randomized Double-Blind Evaluation of a Novel Biotin and Silicon Ingredient Complex on the Hair and Skin of Healthy Women. J Clin Exp Dermatol Res. 12:551.
Received: 31-Dec-2020 Accepted: 14-Jan-2021 Published: 21-Jan-2021 , DOI: 10.35248/2155-9554.21.12.551
Copyright: © 2021 Kalman DS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.