Medical & Surgical Urology
Open Access
ISSN: 2168-9857
ISSN: 2168-9857
Research Article - (2020)Volume 9, Issue 4
The aim of the study was to evaluate the efficacy and safety of Cystone forte tablet in urolithiasis. A randomized, open-label, comparative clinical study was conducted in sixty-two subjects presenting with urolithiasis as diagnosed clinically with calculi measuring 5 to 12mm in size and subjects aged between 18-50 years of either sex. Sixty-two subjects were randomized into trial group and comparator group. Trial group subjects received Cystone forte tablet in the dose of 2 tablets twice daily for a period of 6 weeks while the comparator group received Tamsulosin tablet in the dose of 1 tablet once daily for a period of 6 weeks. The trial group subjects treated with Cystone forte tablets showed comparable results with the improvement of clinical parameters in urolithiasis and with the expulsion of kidney stones as compared to Tamsulosin group. The trial group receiving Cystone forte tablet was found to be effective and was beneficial in the management of urolithiasis. At the end of the study, mild occasional adverse events were reported in both the groups which were resolved. This clinical study demonstrates that, oral administration of Cystone forte tablets at the recommended dose is safe and effective in relieving the symptoms of urolithiasis.
Cystone forte; Calculi; Kidney stones; Renal calculi; Tamsulosin; Urolithiasis
Urolithiasis is the process of deposits or forming stones in the kidney, bladder, and/or urethra (urinary tract). Kidney stones are a common cause of blood in the urine and pain in the abdomen, flank, or groin. Renal calculi formation is an age-old disease and occur in all the socio-economic groups of people and is a major public health issue. Kidney stones occur in 1 in 20 people at some time in their life. Urinary calculi may be silent or may present with varying clinical symptoms and range in different sizes. The incidence of the disease varies in different parts of the world. The etiology of kidney stone disease in all racial groups demonstrate a similarity in the incidence of underlying metabolic abnormalities due to dietary, environmental factors and ethnicity [1].
The incidence of urolithiasis also depends on the geographic area, racial distribution, and socio-economic status of the community. The subsequent changes in dietary habits have affected not only the incidence but also the site and chemical composition of calculi. Calculi featuring mainly calcium oxalate and phosphate is currently more seen in economically developed countries [2].
Many theories have been proposed to explain the mechanism of stone formation. The decrease in urine volume or increase in excretion of stone-forming components such as calcium, oxalate, urate, cystine, xanthine, and phosphate indicate renal calculi development. The epidemiology of renal stones with regard to stone composition all over the world is towards a predominance of calcium oxalate stones. In calculus, uric acid and struvite, reflect the eating habits and infection as risk factors specific to certain population [3]. Calcium oxalate and/or phosphate stones form almost 70% of all renal stones observed in economically developed countries which depends on environmental factors, dietary intake and lifestyle [4].
Type 2 diabetes, obesity, and hypertension are also associated with nephrolithiasis which may be the factor in development of uric acid stones [5]. Calcium oxalate stone formation was significantly associated with several coronary heart disease risk factors, including smoking habit, hypertension, hypercholesterolemia, and obesity [6].
In HIV-infected patients Nephrolithiasis is also a possible adverse event due to the treatment with atazanavir [7].
Several drugs have been used as the treatment option in the past but none has stood at the test of time. Two leading drug classes for medical expulsive therapy are alpha-1-andrenergic receptor blockers and calcium channel blockers other than the invasive therapeutic procedures like extracorporeal shock wave lithotripsy (ESWL), percutaneous nephrolithotomy (PCNL), retrograde intrarenal surgery (RIRS) and laparoscopy.
Urolithiasis treatment strategy involves using medical expulsive therapy (MET) for spontaneous passage of ureteral stones. The 2 leading drug classes are alpha-1-andrenergic receptor blockers and calcium channel blockers. Tamsulosin, an alpha-1-adrenocepter blocking agent, induce spontaneous stone passage by relaxing ureteral smooth muscle tone [8]. Tamsulosin's mechanism of action is by selective relaxation of ureteral smooth muscle, with subsequent inhibition of ureteric spasms and dilatation of the ureteric lumen [9].
Since early days, people wanted to treat kidney stone disease by conservative measures and variety of plant ingredients were used, which lead to an increase in urine volume or reduced pain, or has anti-inflammatory components [10]. Keeping the above factors into consideration, a herbal formulation which can potentially reduce the number of procedures, length of hospital stay, and health care costs is considered due to high need of the hour. Hence, a novel polyherbal formulation, Cystone forte tablet having antilithiatic and lithotriptic, pH renormalizing, diuretic, antimicrobial, antiinflammatory, demulcent, spasmolytic, antioxidant, and tonic actions, was specifically developed for the effective management of urolithiasis due to oxalate stones, phosphate stones, uric acid, urate stones and crystalluria.
The aim of the study was to evaluate the efficacy and safety of Cystone forte tablet in the management of urolithiasis in comparision with Tamsulosin tablet.
Study design
This was a randomized, open-label comparative clinical study designed to evaluate the efficacy and safety of Cystone forte tablet in comparison with Tamsulosin tablet for a period of 6 weeks in the effective management of urolithiasis.
Inclusion criteria
Total of 62 subjects aged between 18 to 50 years presenting with urolithiasis as diagnosed clinically and ultrasonographically with the calculi measuring between 5mm-12mm were considered. Study specific parameters like calcium and uric acid value not more than 2 times the upper limit of normal and willing to sign inform consent document were included into the study.
Exclusion criteria
Subjects with severe obstructive uropathy, serious systemic medical disorder, subjects who have used any drugs for at least 1 week prior to the study, concurrent illness with uncontrolled diabetes and hypertension, with a strong history of food or drug allergy of any kind, subjects on weight reducing diets within 3 months prior to the start of the study, no other drugs (including aspirin) ingested during the course of the study. Pregnant and lactating women were excluded.
Study procedure
This clinical study is approved by independent ethics committee and registered under clinical trial registry India: CTRI/2017/12/010914. Study was initiated with the informed consent process followed by screening of the subjects as per the inclusion and exclusion criteria. All the eligible subjects were randomized to one of the 2 groups: Trial group (Tab Cystone forte) or comparator group (Tab Tamsulosin 0.4 mg). Trial group subject received Cystone forte tablets at a dose of two tablets twice daily and the comparator group received Tamsulosin tablets at a dose of 1 tablet once daily starting from day 1 to end of 6 weeks.
Study subjects were followed up for the clinical assessment on visit days: Visit 0- Screening Visit, Visit 1- At entry visit, Visit 2- At the end of week 2, Visit 3- At the end of week 4, Visit 4- At the end of week 6. All adverse events, either reported or observed by the subjects, were captured in the case report form.
Primary end points
A. Improvement in clinical signs and symptoms of urolithiasis like colicky pain at the loin, hematuria, dysuria, nausea/ vomiting, frequency of micturition, fever.
B. Reduction of calculi size / expulsion of renal calculi as evaluated ultrasonographically.
Secondary end points
A. Safety profile with incidence of adverse events and overall compliance to study drug.
Statistical analysis of the final data was done using paired ‘t’ test and/ANOVA but not limited to these only.
Statistical analysis was done according to intention-to-treat principles. Within and between the group analyses of various parameters from baseline values to 6 week was analyzed using Fisher’s exact test. Statistical analysis was performed using GraphPad Prism software (Version 6.07).
In the present clinical study, total of 62 subjects with an average age range of 37.5 ± 11.3 in Cystone forte group and 40.3 ± 10.6 years in Tamsulosin group (Table 1) presenting with urolithiasis and who completed the study were considered for statistical evaluation. The general physical examination, vitals, hematology and biochemistry were comparable with baseline to end of the study.
Table 1: Clinical assessment of Cystone forte tablets in trial group.
| Demographic Details | ||
|---|---|---|
| Characteristics | Cystone forte Group | Tamsulosin group |
| Number of Subjects | 31 | 31 |
| Gender: No. (%) | ||
| Male | 27 (87%) | 25 (81%) |
| Female | 4 (13%) | 6 (19%) |
| Age, (Mean ± SD), Years | 37.5±11.3 | 40.3±10.6 |
On assessing the values from entry to end of study, Cystone forte group showed statistical significant reduction for colicky pain at the loin, hematuria, dysuria, nausea/ vomiting, frequency of micturition as compared to Tamsulosin group (Table 2).
Table 2: Effect of investigational products on urolithiasis symptoms.
| Parameters | Cystone forte Group | Tamsulosin Group | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Visits | At entry | Week 2 | Week 4 | Week 6 | Visits | At entry | Week 2 | Week 4 | Week 6 | |
| Colicky pain at the loin | Present | 28 | 19 | 9 | 2 | Present | 25 | 19 | 7 | 2 |
| Absent | 3 | 12 | 22 | 29 | Absent | 6 | 12 | 24 | 29 | |
| p value | a: p<0.0159 | a: p<0.0001 | a: p<0.0001 | a: p<0.0001 | ||||||
| Hematuria | Present | 2 | 1 | 1 | 0 | Present | 3 | 1 | 3 | 0 |
| Absent | 29 | 30 | 30 | 31 | Absent | 28 | 30 | 28 | 31 | |
| Dysuria | Present | 10 | 6 | 6 | 1 | Present | 9 | 6 | 5 | 3 |
| Absent | 21 | 25 | 25 | 30 | Absent | 22 | 25 | 26 | 28 | |
| p value | a: p<0.0057 | |||||||||
| Nausea/ vomiting | Present | 5 | 2 | 1 | 1 | Present | 5 | 1 | 1 | 0 |
| Absent | 26 | 29 | 30 | 30 | Absent | 26 | 30 | 30 | 31 | |
| Frequency of micturition | Present | 13 | 10 | 10 | 3 | Present | 12 | 8 | 6 | 4 |
| Absent | 18 | 21 | 21 | 28 | Absent | 19 | 23 | 25 | 27 | |
| p value | a: p<0.0078 | a: p<0.0402 | ||||||||
| Fever | Present | 2 | 1 | 0 | 0 | Present | 2 | 1 | 1 | 1 |
| Absent | 9 | 30 | 31 | 31 | Absent | 29 | 30 | 30 | 30 | |
Fisher's exact test
a: as compared to At entry
Between group not significant
In Cystone forte group, the number of subjects with colicky pain at the loin at entry was 28 which decreased to 2 at the end of the study with a significance of a: p<0.0001. In Tamsulosin group; at entry, 25 subjects had colicky pain at the loin which decreased to 2 subjects at the end of the study with a significance of a: p<0.0001. In trial group, 2 subjects had Hematuria at the entry and it completely resolved at the end of the study, while in comparator group, 3 subjects had Hematuria and was nil at the end of the study. In Cystone forte group, total 10 subjects had Dysuria at entry which decreased to 1 subject at the end of the study with a significance of a: p<0.0057 (as compared to an entry). In Tamsulosin group, total 9 subjects had Dysuria at entry which decreased to 3 subjects at the end of the study with no statistical insignificance.
In Cystone forte group, only 5 subjects had Nausea/Vomiting at the entry which decreased to 1 subject at week 4 and remained same till end of the study, while in Tamsulosin group, only 5 subjects had Nausea/Vomiting at entryand reduced to nil at the end of the study.
In Cystone forte group, total 13 subjects had increased frequency of micturition at entry, reduced to 3 subjects at the end of the study with a significance of a: p<0.0078; while in Tamsulosin group, 12 subjects had increased frequency of micturition at entry, reduced to 4 subjects at the end of the study with a significant of a:p<0.0402.
In Cystone forte group, only 2 subjects presented with fever at entry, and became nil from week 4 onwards till the end of the study. In Tamsulosin group, only 2 subjects presented with fever which decreased to 1 subject at week 2 and remained same till end of the study.
Effect of Cystone forte tablets on number of stones present at entry and week 6
In Cystone forte group, the total number of stones present at entry was 78 (26 at Right kidney, 29 at Left Kidney and 23 at Urinary bladder) which decreased to 45 (13 at Right kidney, 14 at Left Kidney and 18 at Urinary bladder) at the end of the study while in Tamsulosin group, the total number of stones present at entry was 89 (39 at Right kidney, 34 at Left Kidney and 16 at Urinary bladder) which decreased to 27 (12 at Right kidney, 12 at Left Kidney and 3 at Urinary bladder) at the end of the study (Table 3, Figure 1).
Table 3: Effect of investigational products on number of stone present.
| Cystone Forte Tablet | At Entry | Week 2 | Week 4 | Week 6 |
|---|---|---|---|---|
| Right Kidney | 26 | 22 | 19 | 13 |
| Left Kidney | 29 | 28 | 19 | 14 |
| Urinary bladder | 23 | 25 | 20 | 18 |
| Total | 78 | 75 | 58 | 45 |
| Tamsulosin Tablet | At Entry | Week 2 | Week 4 | Week 6 |
| Right Kidney | 39 | 28 | 14 | 12 |
| Left Kidney | 34 | 23 | 18 | 12 |
| Urinary bladder | 16 | 17 | 8 | 3 |
| Total | 89 | 68 | 40 | 27 |
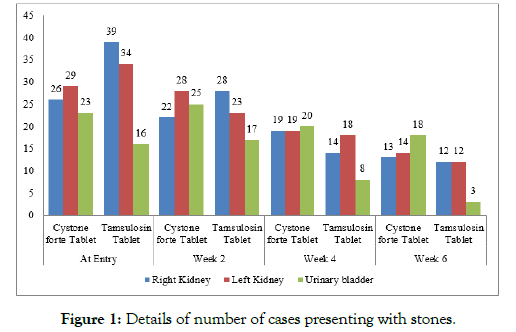
Figure 1: Details of number of cases presenting with stones.
Effect of Cystone forte tablets on Calculus Expulsion
In Cystone forte group, overall calculus present at screening was 31, out of which 12 calculus were expelled at the end of the study. In Tamsulosin group, 31 calculus were present at screening, out of which 15 calculus got expelled at the end of the study.
Of the above, the number of calculus size <7 mm present at screening was 12 in Cystone forte group where 6 stones got expelled at the end of the study. In Tamsulosin group, the number of calculus size <7 mm present at screening was 12, out of which 8 stones were expelled at the end of the study.
The number of Calculus of size >7 mm present at screening was 19 in Cystone forte group where 6 stones got expelled at the end of the study. In Tamsulosin group, the number of calculus of size >7 mm present at screening was 19 stones, out of which 7 stones were expelled at the end of the study (Table 4, Figures 2 and 3). Effect of Cystone forte tablets on Calculus Expulsion in percentage In Cystone forte group, 50% of the calculus of the size <7 mm were expelled, 32% of the calculus of the size >7 mm were expelled at the end of the study.
Table 4: Effect of investigational products on calculus expulsion.
| Overall Calculus Expelled | Cystone forte Group | Tamsulosin Group |
|---|---|---|
| Stone Present at Screening | 31 | 31 |
| Stone Present at Week 6 | 19 | 16 |
| Stone Expelled at Week 6 | 12 | 15 |
| Calculus size <7 mm | ||
| Stone Present at Screening | 12 | 12 |
| Stone Present at Week 6 | 6 | 4 |
| Stone Expelled at Week 6 | 6 | 8 |
| Calculus size >7 mm | ||
| Stone Present at Screening | 19 | 19 |
| Stone Present at Week 6 | 13 | 12 |
| Stone Expelled at Week 6 | 6 | 7 |
Descriptive Statistics (Count), Value Represented in Number of Subjects
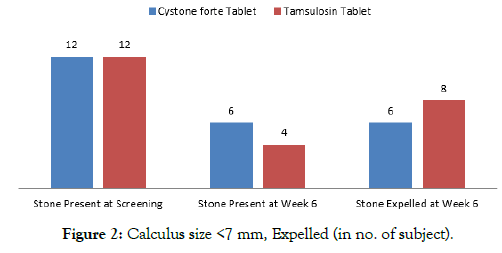
Figure 2: Calculus size <7 mm, Expelled (in no. of subject).
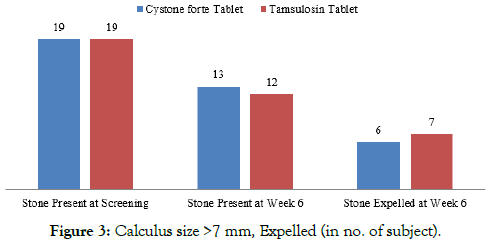
Figure 3: Calculus size >7 mm, Expelled (in no. of subject).
In Tamsulosin group, 67% of the calculus of the size <7 mm were expelled, 37% of the calculus of the size >7 mm were expelled at the end of the study (Table 5, Figure 4).
Table 5: Effect of investigational products on calculus expulsion in percentage.
| Stone Expelled at Week 6 | Cystone forte group | Tamsulosin group |
|---|---|---|
| Calculus size <7 mm | 6/12 (50%) | 8/12 (67%) |
| Calculus size >7 mm | 6/19 (32%) | 7/19 (37%) |
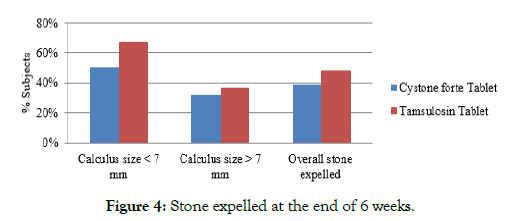
Figure 4: Stone expelled at the end of 6 weeks.
Adverse events
In Cystone forte group, total of 6 subjects experienced adverse events like, Constipation, Dizziness, Abdominal discomfort/ distension, flatulence and acidity which were non-serious and mild in intensity and were resolved without sequelae.
In Tamsulosin group, total of 10 subjects experienced dizziness, flatulence, acidity, headache and diarrhea which were mild in intensity and abdominal discomfort/distension were non-serious and of mild to moderate in intensity which got resolved without sequelae.
Urolithiasis is a very common clinical problem with varied symptomatology. Cystone Forte tablet is a polyherbal formulation and principal herbs include: Didymocarpus pedicellata which is well known for its antilithic activities; and used for stones in kidney and bladder [11]. The nephroprotective activity of the plant is attributed to polyphenolic compounds [12]. Saxifraga ligulata has astringent, tonic and spasmolytic; used in dysuria, and other urinary disorders and are widely used in renal stones as it has the lithotriptic activity [13]. Alcoholic extracts is found to be useful as curative in dissolving the calculi developed by foreign body insertion method using zinc discs in the bladder of albino rats against phosphate type of Urolithiasis [14]. Rubia cordifolia roots are rich in anthraquinones and their glycosides, with most important are purpurin, munjistin has blood purifier, astringent, diuretic, and antiseptic activities [15]. Extract of Rubia cordifolia (Root) shows antimicrobial activity against Eischeria coli, Bacillus subtilis, Klebsiella pneumoniae, Proteus vulgaris, and Psuedomonas aeruginosa. In another study, some antimicrobial actives, emodin and physcion were isolated as the most active constituent [16]. An essential oil from tuber of Cyperus scarious contains sesquiterpine, cyperenone, and shows antibacterial, anti-inflammatory activities [17]. The plant has diuretic, and astringent activities; and used in urinary disorders [18]. Achyranthes aspera contains alkaloid achyranthine; used in kidney stone, minor hemorrhage. It also has wound healing promoter activity [19]. The plant is used in various clinical trials due to its diuretic, antispasmodic, and antiallergic activities [20]. Onosma bracteatum has cooling, astringent, and diuretic activities; and used in dysuria, and urethral discharges [21]. The plant has demulcent activity and is used for the management of urinary bladder irritation [22]. Vernonia cinerea plant is used to relieve the spasm and strangury in bladder [23]. Aerial parts give 7-monobeta-D-glucopyranoside. Whole plant contains beta amyrin triterpene, lupeol acetate, beta amyrin and potassium chloride. This is used specially for dysuria, spasm of bladder, strangury and in mild hemorrhage due to styptic activity [24]. Results indicate that the extracts could possess analgesic, antipyretic and anti-inflammatory properties [25]. Shilajeet (Purified) is used in various disorders due to multifocal therapeutic activities. It promotes strength and useful in the treatment of obstinate urinary disorders, urinary calculi, spasmodic pain. It is generally used in the management of stone in urinary tract [26]. Classics refer about the use of Shilajeet (Purified) has influences on endocrine, automatic, and brain functional changes [27]. Hajrul yahood bhasma has lithotriptic property and potent diuretic action [28].
Various Pre-clinical studies showed beneficial effects of Cystone forte. Cystone forte was evaluated for its efficacy in experimental models of urolithiasis namely glycolic acid, oxamide and calculi implantation (human urinary calculi implanted in the urinary bladder) etc. Cystone treatment at dose levels of 500 and 750 mg/kg body weight showed a significant reversal of glycolic acidinduced changes in urine and renal parameters. The observations in three experimental models of urolithiasis clearly support the antilithic and lithotriptic property of Cystone.
Cystone forte was evaluated using in vitro and in vivo models for its effect on mineralization and demineralization reaction, which is an important factor in renal calculi formation. In vitro studies with Cystone showed a decrease in calcium and phosphate contents by inhibiting their deposition, subsequent growth and stimulating the dissolution of mineral phase. Studies conducted on urine samples of animals administered orally with Cystone inhibited the precipitation of calcium and phosphate as well as the growth of the preformed mineral phase.
Cystone forte was investigated for its protective effect in cisplatininduced nephrotoxicity. It has been suggested that the renal toxicity of cisplatin is implicated to the oxygen free radicals. Cystone inhibited the lipid peroxidation in renal cortical slices thus suggesting the antioxidant property, which in turn ameliorated cisplatin-induced renal damage. Cystone treatment also alleviated the serum BUN and creatinine levels, which was increased in the cisplatin treated animals. In addition, Cystone treatment also improved the urinary creatinine clearance in animals as compared to cisplatin-administered group.
From various experimental studies, the Cystone attributed to possess diuretic property, prevents supersaturation of lithogenic substances, controls oxamide absorption from the intestine, corrects crystalloid-colloid imbalance, causes disintegration of the calculi and the crystals by acting on the mucin, which binds to particles together, inhibits calculogenesis by its anti-infective and anti-inflammatory properties and alleviates symptoms of burning and pain on urination.
Previous clinical studies of Cystone forte in the management of urolithiasis showed significant decrease in the presence of renal calculi and its size after treatment with Cystone forte. Significant improvement was seen in clinical symptoms like clearance of calculi, symptomatic relief, and increased urine volume with no significant adverse events during the clinical study. Additionally, it was also found to be beneficial in expelling and/or reducing the size of the renal stones, including bigger-sized stones ranging from 7 to 12 mm [29].
Tamsulosin is recommended for patients receiving a diagnosis of a ureteral stone, who do not require immediate urologic intervention. The drug Tamsulosin improves stone passage in patients with stone size ranging 5 to 10 mm. In a systematic review and meta-analysis of 8 randomized, double-blind, placebo-controlled trials, found that Tamsulosin improves stone passage in a subgroup of participants with large distal ureteral stones [30].
Present clinical study observed a reduction in the total score for clinical parameters, at the end of the study in both the groups. Furthermore, all the haematological and biochemical parameters were within the normal limits with no significant differences observed in both the groups before and after the treatment. Overall response of subjects to the treatment was good. There were few mild adverse events, which got resolved without any clinical sequelae at the end of the study. The Overall Impression about treatment by the study investigator was found to be comparable with no significant difference between the Cystone forte and Tamsulosin groups.
The present report demonstrated that subjects treated with Cystone forte tablets showed improvement in clinical parameters of urolithiasis and in the expulsion of kidney stones and was comparable to the standard comparator Tamsulosin tablet. From the results, it can be understood that, the subjects treated with Cystone forte tablets was found comparable with the existing standard comparator Tamsulosin tablet in the management of urolithiasis. The study results indicate that the poly herbal formulation Cystone forte tablet, is safe and effective in the treatment of urolithiasis, with significant improvement in clinical symptoms like colicky pain at the loin, dysuria, nausea/ vomiting, hematuria and in the clearance of both <7 mm and >7 mm calculi. The overall response to decrease in the symptoms associated with the urolithiasis was found to be good. Few mild and occasional adverse events were seen in some subjects in both the groups; however, it was resolved without any clinical sequelae at the end of the study. The efficacy of Cystone forte tablets can be attributed as due to the potential synergistic actions of the potent herbs and minerals present in the formulation. The overall compliance to the treatment was good. Additionally, it is also found to be beneficial in expelling and/or reducing the size of the renal stones, including bigger-sized stones ranging >7mm. With all these findings, the polyherbal formulation Cystone forte, can be a good alternate to invasive and modern therapy in the unmet medical need of Urolithiasis. Hence, this clinical study demonstrates that oral administration of Cystone forte tablets is beneficial and effective for the management of urolithiasis.
Citation: Chaudhari RR, Shah PM, Shetty AR (2020) A Randomized, Open label, Comparative Clinical Study to Evaluate the Efficacy and Safety of Cystone Forte Tablet in Urolithiasis. Med Surg Urol. 9:4. Doi: 10.24105/2168-9857.9.235
Received: 16-Jul-2020 Accepted: 17-Sep-2020 Published: 24-Sep-2020 , DOI: 10.35248/2168-9857.20.9.235
Copyright: © 2020 Chaudhari RR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.