Journal of Hepatology and Gastrointestinal disorders
Open Access
ISSN: 2475-3181
ISSN: 2475-3181
Research Article - (2019)Volume 5, Issue 1
Introduction: Ulcerative Colitis (UC) is a chronic inflammatory condition of the colon affecting 5 million patients globally. Despite the recent clinical advances in drug therapies, patients continue to have exacerbations of UC. Alterations in gut microbiota, more specifically reduced intestinal microbial diversity, have been found to be associated with relapse of UC. Therefore therapeutic strategy targeting the gut microbiota has the potential to induce remission in UC. This study was done to evaluate the efficacy and tolerability of synbiotic as an adjuvant to standard therapy in patients with UC and to evaluate the ability of synbiotic in preventing relapse and prolonging the remission of UC.
Methods: An interventional open label, randomized, comparative clinical trial was done with 32 patients. Patients were randomized into two groups, Study group (n=16) received synbiotic one capsule b.d along with standard therapy. Group B (n=16) received standard therapy only. Statistical analyses of the efficacy and safety parameters were done using chi square test paired t test and ANOVA.
Results: In our study, at the end of 6th month, statistically significant number of patients had remission in study group when compared to control group, with p<0.05. Significantly higher number of patients in control group had relapse compared to study group and also there was a statistically significant reduction in steroid intake in study group.
Conclusion: From this study we can conclude that synbiotic therapy along with standard treatment is effective in inducing and maintaining remission with a reduction in steroid dosage in UC and tolerable.
Ulcerative colitis; Synbiotic therapy; Probiotics; Steroids; Remission; Relapse; Gut microbiota
Ulcerative colitis (UC) is characterized by relapsing and remitting episodes of inflammation limited to the mucosal layer of the colon [1]. The incidence of UC is rising with time, with prevalence 44.3 per 100,000 in India [2]. In UC 15% of the patients have a life time risk to develop severe exacerbations requiring hospitalization 67% of patients have at least one relapse within 10 years 48.1% of the patients who had taken standard therapy experience relapse in 12 months [3,4]. Twenty to thirty percent of patients with ulcerative colitis will require colectomy for acute complications or for medically intractable disease. Incidence of Colorectal cancer is 2.5 percent after 20 years and 7.6 percent after 30 years of disease [3-5].
The aim of treatment of UC is induction of remission, prevention of relapses, mucosal healing, the avoidance of colectomy, and decreasing the likelihood of the development of cancer. Preparations such as 5 aminosalicylates (5-ASA) preparations are effective in inducing and maintaining remission in approximately 75% of the patients [6]. In patients who fail to respond to 5-ASA therapy, oral corticosteroids like prednisone are used to induce remission and are not used for maintenance due to lack of efficacy and adverse effects [7]. Only 60% of the patients with severe attacks of ulcerative colitis respond to corticosteroids. Immunomodulation therapy is of great benefit in such patients to attain remission. Azathioprine, mercaptopurine, methotrexate, cyclosporine, or tacrolimus are the most commonly used immunosuppressive drugs [8]. In genetically susceptible host, aberrant immune response and dysregulation of mucosal immune system has been implicated in the pathogenesis of UC [9]. Alterations in gut microbiota, and specifically reduced intestinal microbial diversity, have been found to be associated with chronic gut inflammation. Patients with UC have a reduced abundance of Lactobacillus spp., Bacteroides spp. and Eubacterium spp. [7].
Patients with UC are very often refractory to conventional therapy and therefore require the use of alternative therapies. Evidence of dysbiotic intestinal microbiota as the key component in the pathogenesis of UC suggests the need of developing new therapies targeting intestinal bacteria [10]. In this context, synbiotic is a novel approach where probiotics and prebiotics are combined in an attempt to obtain synergistic effects and faster colonization of useful bacteria in the gut. In this study, we have used synbiotic containing patented strains of Lactobacillus sporogenes (probiotic), Streptococcus faecalis T-110, Clostridium butyricum TO-A and Bacillus mesentericus TO-A (pre and probiotic).
Lactobacillus sporogenes T-110 produces lactic acid which inhibits the growth of pathogenic bacteria. Streptococcus faecalis T-110, Clostridium butyricum TO-A and Bacillus mesentericus TO-A help in the proliferation of 32 sp. of Bifidobacterium by production of a nutrient 3, 3 Dihydroxy Azetidine. Clostridium butyricum TOA yield short chain fatty acids such as butyric acid and acetic acid with a resultant decrease in intestinal pH and inhibition of growth of harmful bacteria and also help to regularize abnormal bowel movements [11].
Probiotics exert their actions via multiple mechanisms such as inhibiting the growth of pathogenic bacteria, regulating the bowel movement and strengthening the gut immunity. Synbiotic therapy helps in reduction of inflammation in the colonic mucosa, regeneration of colonic epithelial tissue and improvements in inflammatory markers in patients with active UC. Studies have shown that synbiotics can be considered as a valid therapeutic option in adjuvant to conventional therapy to induce and maintain remission in UC with minimal side effects [12]. Therefore this study was done to evaluate the efficacy and tolerability of synbiotic in the treatment of ulcerative colitis.
• To evaluate the efficacy and tolerability of synbiotic as an adjuvant to routine therapy in patients with UC.
• To evaluate the ability of synbiotic in extending the duration of remission of UC.
This study was conducted in patients suffering from ulcerative colitis attending the Department of Gastroenterolgy, Madras Medical College, Chennai, after the approval from Institutional Ethics Committee.
A total of 32 patients diagnosed with UC within one year were included and randomized into two groups, Study group(n=16) received synbiotic one capsule twice daily along with standard therapy (Sulphasalazine 250 mg to 500 mg two tablets 3 times a day and Prednisolone 40 mg once a day) and control group (n=16) received only standard therapy. Each synbiotic capsule contained Streptococcus faecalis T-110 JPC: 60 million, Clostridium butyricum TO-A: 4 million, Bacillus mesentricus TO-A JPC:2 million, Lactobacillus sporogenes: 100 million. Male or female patients in the age range of 18 to 60 years diagnosed with UC within one year and willing to give informed consent were included in the study. Pregnant or lactating women, Patients diagnosed with infectious, ischemic, radiation or chemical induced colitis; UC with complications; Patients diagnosed with malignancy, thromboembolic disease; Patients with history of complications in previous abdominal surgery; Patients with history of antibiotic administration in the past one month; Patients with history of use of synbiotic/probiotic; Patients with hypersensitivity for Probiotics/Synbiotics; Patients with chronic or severe respiratory, cardiovascular, CNS, endocrine and other gastrointestinal disorders were excluded.
Study was conducted for a period of 6 months which included 3 months of intervention and 3 months of follow up. The assessment of efficacy was done using the Truelove and Witts classification of UC at the start of the study and every month until the end study for 6 months. The duration of remission is assessed by the number of relapses in each patient during the study period and follow up period. The safety and tolerability of the Synbiotic was assessed by adverse event profile. Prednisolone is tapered by 5 mg per week until the dosage is 20 mg per day. This dosage is then tapered by 2.5 mg per week until the drug is discontinued. Clinical examination and laboratory investigations were done. Colonoscopy was done only if it was not done already to confirm the diagnosis.
Statistical analysis
Statistical analyses of the efficacy and safety parameters were done using chi square test, paired t test and ANOVA.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
This study included 32 patients with each study and the control group having 16 patients. All the baseline characteristics were similar in both groups and with respect to age, gender severity of the disease and lab findings and both the groups were comparable with no statistically significant difference between them. Assessment of severity of the disease was done by Truelove and Witts classification. At the end of 3rd month, the severity score of the disease reduced from moderate (62.5%) and severe (37.5%) to mild (12.5%) and remission (87.5%) in study group. It did not show a statistically significant difference both within the groups and between the groups. (p=0.394, 0.696). The severity score also decreased in control group from moderate (68.75%) and severe (31.25%) to mild (31.25%) and remission (68.75%) without statistically significant difference within the groups and also between the groups (p:0.394, 0.611 respectively) (Figure 1). But at the 6th month there was a statistically significant reduction in the severity of the disease shown by reduction from moderate (62.5%) and severe (37.5%) to mild (0%) and remission (100%) in synbiotic group with a p value of <0.001. In the control group, there is no significant reduction in the severity of the disease with a p value of 0.611 (Figure 1).
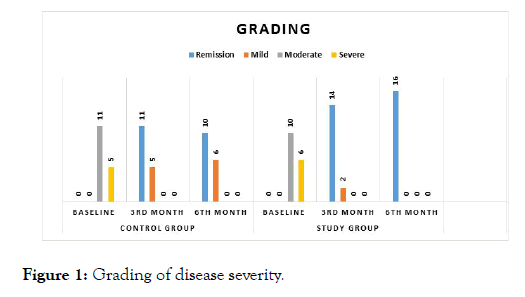
Figure 1: Grading of disease severity.
During intervention period and follow up period, higher number of patients in control group had relapse compared to study group patients which was also statistically significant (Figure 2). There was a statistically significant reduction in steroid intake in study group when compared to control group at the end of 3rd and 6th month. In patients of study group, steroid requirement was nil at the end of 3 months (Figure 3).
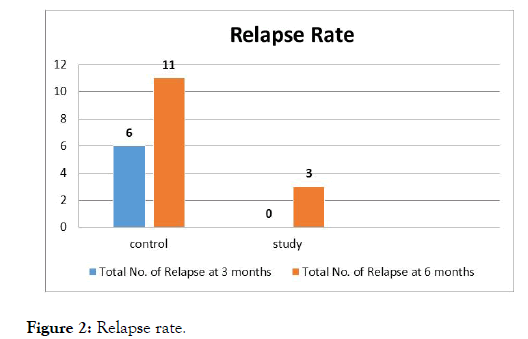
Figure 2: Relapse rate.
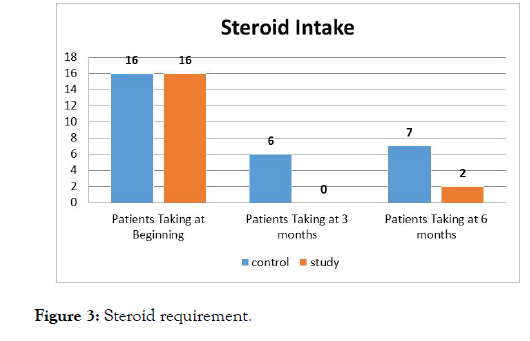
Figure 3: Steroid requirement.
The increase in haemoglobin and decrease in ESR were statistically significant in both the groups from baseline to the end of 3 months and 6 months with p<0.05. All other hematological and biochemistry laboratory findings were not of any statistical significance over the course of the study in either study group or control group.
Ulcerative colitis is characterized by severe exacerbations and remissions due to change in treatment, super infections, illness, stress or spontaneous remission [13]. Patients with ulcerative colitis experience both emotional and economic burden, reduced quality of life and loss of work days. The direct and indirect health care costs are astoundingly high [14]. The exacerbated immune response produces remissions and relapses and hence the disease requires lifelong medication to maintain remission and to prevent relapses. The current treatment strategy consists mainly of 5-amino salicylic acid and steroids. But 5-ASA preparations are associated with side effects like headache, nausea, abdominal pain and cramping, loss of appetite, vomiting, rash, or fever and prolonged use of steroids is not recommended due to development of glucose intolerance, osteoporosis, susceptibility to infection, myopathy [15]. Synbiotic are synergistic combination of probiotic (viable beneficial bacteria) and prebiotic (selective substrate to improve the growth of bacteria). Synbiotic acts by increasing the nonpathogenic bacteria in the gut and also decrease the production of IL-I and TNF-α in gut mucosa which play a major role in the inflammation produced in Ulcerative Colitis and by many other mechanisms [16].
A recent study has reported that synbiotic therapy resulted in improvements in inflammatory markers, reduced inflammation in the rectal mucosa and regeneration of colonic epithelial tissue in patients with active UC [17].
In our study, the age of the patients in both study groups range between 18 years to 60 years, this is in accordance with the occurrence of UC, which is usually in the third or fourth decade of life. This is similar to the age group in the study conducted at University of Dundee [17]. Among the 32 patients, 68.75% (n: 22) of patients were males and 31.25% (n:10) of patients were females.
The severity of the disease, hematological and biochemical parameters, colonoscopy findings and the biopsy findings were similar in both groups at baseline. According to Truelove and Witts classification of assessment of severity findings in this study, at the end of 3rd month, number of patients who attained remission in both Study group and control group were not statistically significant. This may be due to the smaller sample size. But at the end of 6th month, statistically significant number of patients had remission in study group when compared to control group, with p<0.05 (Figure 1). This is in accordance with a randomized controlled trial conducted by Shunji Fujimori, Katya Gudis in 09 February 2009, on the efficacy of synbiotic versus probiotic or prebiotic treatment to improve the quality of life in patients with Ulcerative Colitis which had showed the similar effect [18].
These findings suggest that the remission induced by synbiotic lasts for 6 months and also synbiotic continue to have beneficial effect atleast for 3 months after discontinuation of treatment. No relapses occurred over the first 3 month period in study group as against 6 relapses (37.5%) in the control group which showed a statistically significant difference with a p value of 0.018. During the follow-up period there were 3 relapses (18.75%) in the study group which is much lower than the control group which had 11 relapses (68.75%) and showed a statistically significant difference with a p value of 0.011 (Figure 2). The lesser the relapses indicates the longer the duration of remission. Our study shows a remission of about 90% in comparison with 5-amino salicylic acid in a Randomized Controlled Trial where the remission rate was only 39% to 62% [19].
There was identical improvement of the hemoglobin levels in both the groups and the identical reduction in the ESR in both the groups. Improvement in haemoglobin levels is mainly because of the reduction in the symptoms of the bloody diarrhea and thereby improving the anemic status.
The tolerability of synbiotic was assessed by adverse event monitoring and also by other hematological and biochemical parameters like platelets, total count, blood sugar, urea, serum creatinine, SGOT, SGPT, total protein, albumin, bilirubin, serum electrolytes, ultrasound abdomen at baseline and 3rd month. All these parameters did not show any difference between the study groups. Two patients in study group complained of bloating sensation in abdomen which got relieved by reassurance and did not warrant discontinuation of medication. No other adverse effects were seen in the entire study group. On analyzing the results of the two study groups, the combination of synbiotic with standard therapy proves its efficacy in inducing remission over 6 month period. It is also efficacious in maintaining the remission period during drug intake and also during follow up period. Synbiotic also decreased the steroid intake in patients with severe and moderate disease (Figure 3). Hence this study shows the effectiveness of synbiotic in Ulcerative Colitis but it has minimal side effects. Small sample size is one of the limitations of our study, other being short duration of the study. Further large scale trials of longer duration are necessary to prove the long term benefits of symbiotic in patients with ulcerative colitis.
In conclusion, synbiotic therapy along with standard treatment is effective in inducing remission and in maintaining remission with a reduction in steroid dosage. Synbiotic is well tolerated and is safe to be used in patients with ulcerative colitis.
Citation: Malathi K, Nandini R, Dhanasekar KR, Shilpa BN (2019) A Randomized Open Label Study to Evaluate the Efficacy and Tolerability of Synbiotic in the Treatment of Ulcerative Colitis. J Hepatol Gastroint Dis 10:165. doi: 10.35248/2475-3181.19.5.165
Received: 13-Dec-2018 Accepted: 13-Feb-2019 Published: 18-Feb-2019
Copyright: © 2019 Malathi K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.