
Journal of Clinical Trials
Open Access
ISSN: 2167-0870

ISSN: 2167-0870
Research Article - (2022)Volume 12, Issue 3
Background: The inactivated COVID-19 vaccine developed by Sinovac (CoronaVac®) has been shown to effectively prevent Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infections. Phase I/II clinical trials in healthy adults aged 18-59 and over 60 showed good safety and immunogenicity in China. Phase III clinical trials are currently being carried out in Brazil, Indonesia, Chile, and Turkey. The WHO approved CoronaVac® for emergency use in adults on June 01, 2021. A Phase I/II clinical trial in children and teenagers aged 3-17 was performed in China in 2020.
Methods: This is a global multi-center, randomized, double-blinded, and placebo-controlled phase III clinical trial that aims to assess the safety, efficacy, and immunogenicity of CoronaVac® in the pediatric population. This vaccine will be administered to infants, children, and adolescents aged 6 months to 17 years and compared to a placebo. Five countries will participate in this study, South Africa, Malaysia, Kenya, the Philippines and Chile. This report will focus on the study to be performed in Chile. Volunteers will randomly receive two intramuscular doses of CoronaVac® or the control comparator (placebo), in a 1:1 ratio, with an interval of 28 days between each dose. The study will be finished after the last visit of the last volunteer. Efficacy assessments include the surveillance of COVID-19-like symptoms, clinical laboratory confirmation of SARS-CoV-2 infection by RT-PCR, and recording of COVID-19-related hospitalizations. Safety assessment considers monitoring Adverse Events (AEs) within 30 minutes after administration of each dose and monitoring and collecting of Adverse Events of Special Interest (AESI) and Serious Adverse Events (SAEs) until 12 months after the first dose. Immunogenicity assessments will focus on neutralizing and anti-Spike protein antibodies and will be performed in a sub-group of volunteers. Any confirmed COVID-19 case will be followed until resolution. Total RNA of saliva samples will be obtained from confirmed COVID-19 cases for viral genome sequencing to detect SARS-CoV-2 circulating variants of concern.
Discussion: Vaccination of children and teenagers will be a crucial step in controlling the spread of COVID-19 and will also be fundamental to prevent the emergence of syndromes such as Long-COVID and PIMS-TS.
SARS-CoV-2; COVID-19; CoronaVac®; Inactivated vaccine; Pediatrics; Clinical trial; Randomized controlled trial
SARS-CoV-2 is responsible for the current pandemic of Coronavirus Disease 2019 (COVID-19) [1,2]. This disease was described for the first time in Wuhan, China, in December of 2019 [3]. Since then, SARS-CoV-2 has caused almost 240 million cases of infection and nearly 5 million deaths around the world [3]. The urgent and mandatory development of effective therapies against this virus has led to 7 vaccines being approved for emergency use by the WHO [4]. Most approved SARS-CoV-2 vaccines express or encode either the Spike (S) protein or the Receptor-Binding Domain (RBD) of this protein to induce an immune response against it [5,6]. Since the S protein is responsible for binding to the hACE2 receptor on the target cell, neutralizing this viral component with circulating antibodies should decrease the infection of SARS-CoV-2 [5,6]. However, mutations of this protein by circulating variants of concern of this virus could impact the neutralizing capacities of induced antibodies [4,7]. Whole virus inactivated platforms are among the earliest vaccines ever used to prevent diseases [5]. For SARS-CoV-2, inactivated vaccines include more antigens than just the S protein; therefore, they might protect against circulating variants of concern through recognition of several viral antigens [5,8].
CoronaVac® is an inactivated COVID-19 vaccine (generated in Vero cells) developed by Sinovac Life Science Co. Ltd., which could induce an active immunity to prevent the disease caused by SARS-CoV-2 [9-12]. Pre-clinical studies show that this vaccine was safe and induced an immune response against this virus in mice, rats and rhesus macaques [9]. Phase I/II clinical trials were held in the Jiangsu Province, China, in healthy adults aged 18 to 59. Phase I/II clinical trials with elderly aged 60 years and over were held in the Hebei Province, China. Phase I/II clinical trials in children and adolescents aged 3 to 17 were launched in the Hebei Province, China, on October 31, 2020. These clinical trials have completed the safety and immunogenicity evaluation. The results showed good safety and immunogenicity in adults, the elderly, children, and adolescents [10-12]. Four phase III clinical trials for this vaccine are being carried out in Brazil (18 years old and above), Indonesia (18-59 years old), Chile (18-59 and over 60 years old), and Turkey (18-59 years old). These trials used the same randomized, double-blind, placebo-controlled study design to evaluate the efficacy of the vaccine [13-19]. The results from Brazil showed good efficacy and safety for this vaccine. The positive results led to conditional marketing approval in China on February 5, 2021 (approval number: 2021S00156, 2021S00157). The WHO approved CoronaVac® for emergency use in adults on June 01, 2021 [20]. Accordingly, this vaccine was approved in Chile for emergency use in adults on January 20th, 2021, and in 6-17 years old children and adolescents on September 6th, 2021 [21].
This protocol was proposed to further evaluate the safety of CoronaVac® in children and adolescents in populations different from the Chinese and assess its efficacy in this population. To this date, only the BNT162b2 vaccine is approved by the U.S. FDA to be used in the pediatric population younger than 12 years, even though that population is still vulnerable to COVID-19 [22]. CoronaVac® has shown promising protective efficacy in the pediatric population aged from 3 to 17 years. A high vaccination rate in this population will likely be essential to reach an immunologic barrier that reduces the morbidity and mortality of COVID-19 [12]. The overall study aims to evaluate the efficacy and safety of CoronaVac® among children aged 6 months to 17 years, which also expands the age range initially approved for this vaccine. This study considers placebo as a control to compare the frequency and magnitude of clinical and immunological endpoints changes. Randomization and blinding of volunteers will be considered to reduce bias in the assignment of the two groups and during data collection and evaluation of study endpoints, respectively. Given the potential risk of disease enhancement reported for pediatric populations [23], additional surveillance for COVID-19 will be considered in this study. Finally, considering the possibility of approval for emergency use of this vaccine in the pediatric population, the aims of this study may be modified in the future.
Objectives of this trial
These objectives may be modified upon approval of emergency use of this vaccine in the pediatric population in the corresponding countries.
Primary objective: To evaluate the efficacy of two doses of CoronaVac® against RT-PCR confirmed symptomatic COVID-19 cases in volunteers aged 6 months to 17 years.
These objectives will be modified accordingly to the changes in local vaccination plans and regulations.
Efficacy secondary objectives:
• To evaluate the efficacy of at least one dose of CoronaVac® against RT-PCR confirmed symptomatic COVID-19 cases.
• To assess the effect of two doses of CoronaVac® against RTPCR confirmed symptomatic COVID-19 cases in baseline SARS-CoV-2 uninfected volunteers.
• To evaluate the efficacy of two doses of CoronaVac® on hospitalization/death caused by COVID-19.
These objectives will be modified accordingly to the changes in local vaccination plans and regulations.
Safety secondary objectives:
• To evaluate the safety of CoronaVac® in terms of solicited local and systemic AEs during seven days after each dose vaccination and in terms of unsolicited AEs for 28 days postvaccination (Subgroup). • To evaluate safety in terms of SAEs. • To evaluate safety in terms of AESI.
Immunogenicity secondary objective: To evaluate the immune response induced by CoronaVac® in a subgroup of volunteers compared to placebo. In Chile, the humoral immune response will be evaluated to determine circulating antibodies against different proteins of this virus. The cellular immune response will be evaluated upon stimulation of Peripheral Blood Mononuclear Cells (PBMCs) with megapools of peptides derived from the proteome of SARS-CoV-2.
Trial characteristics
Participants: As seen in Figure 1, 14,000 healthy volunteers aged 6 months to 17 years of both genders will be enrolled and randomly assigned into the vaccine or placebo group for the entire global study. In Chile, 4,000 volunteers aged 3 to 17 years will be recruited, and volunteers aged 3 to 5 will be randomly assigned to the vaccine or the placebo group. Pregnancy and breastfeeding will be exclusion criteria, and confirmed pregnancy during the study will lead to the discontinuation of the volunteer in the study. Accordingly, appropriate follow-up visits or medical care will be arranged with the agreement of the volunteer. Other exclusion criteria are a history of confirmed infection of SARS-CoV-2 before randomization, immunosuppression, autoimmune diseases, bleeding disorders, or any unstable chronic disease. All volunteers or their guardians will sign a voluntary Informed Consent Form and undergo a medical screening evaluation to confirm that all the inclusion and exclusion criteria are met.
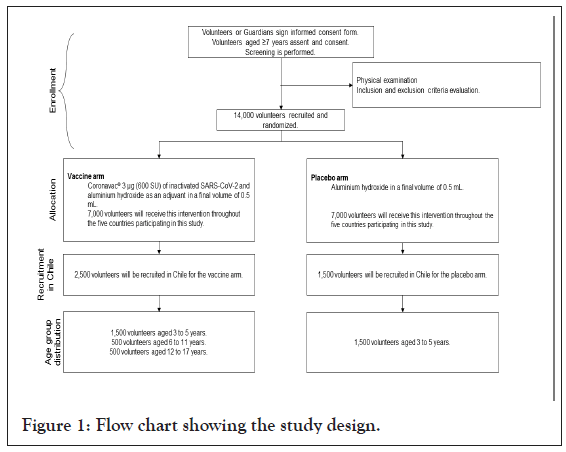
Figure 1: Flow chart showing the study design.
Intervention and comparator
Experimental intervention: CoronaVac® was manufactured in Beijing, China, by Sinovac Life Sciences. This vaccine contains 3 μg (600 SU) of inactivated SARS-CoV-2 (strain derived from CN02 grown in Vero 25 cells) and aluminum hydroxide as an adjuvant in a final volume of 0.5 mL.
Control comparator: The placebo used in this study consists of aluminum hydroxide in a final volume of 0.5 mL. The experimental intervention and placebo schedule will be administrated in two 0.5 mL doses intramuscularly (deltoid) in a four-week interval.
Main outcomes
The primary endpoint is the incidence of RT-PCR confirmed symptomatic COVID-19 cases with onset at least 14 days after the second dose. Secondary efficacy endpoints are the incidence of RTPCR confirmed symptomatic COVID-19 cases with onset at least 14 days after the first dose, the incidence of RT-PCR confirmed, symptomatic COVID-19, with onset at least 14 days after the second dose in SARS-CoV-2 uninfected volunteers, and the incidence of hospitalization/death caused by COVID-19 with onset at least 14 days after the second dose. Of note, any COVID-19 confirmed case will be monitored until resolution.
Whole viral genome sequencing will be performed in all confirmed samples to evaluate outstanding mutations related to circulating variants of concern in local laboratories for each country. Secondary safety endpoints are the occurrence, intensity, duration, and relationship of solicited local and systemic AEs during seven days following each dose vaccination and of unsolicited AEs for 28 days post-vaccination, the occurrence and relationship of SAEs (from the first dose to 12 months after the last dose), and the occurrence and relationship of AESI (from the first dose to 12 months after the last dose). Secondary immunogenicity endpoints are the analysis of SARS-CoV-2 neutralizing antibody titers by micro-cytopathic method and the analysis of anti-Spike SARS-CoV-2 antibodies by electrochemiluminescence immunoassay.
Exploratory immunogenicity endpoints consider the following: Analysis of cytokine secreting SARS-CoV-2 specific T cells by ELISPOT; Analysis of activated and memory SARS-CoV-2 specific T cells by flow cytometry; Analysis of circulating IgG specific to SARS-CoV-2 proteins other than the S protein; Analysis of circulating IgG specific against the S protein of SARS-CoV-2 in saliva.
Randomization and blinding (masking)
This study will be implemented with central randomization. Volunteers will be randomly assigned to the vaccine or placebo arm in a 1:1 ratio. In Chile, volunteers aged 6 years and over will not receive a placebo. Randomization will be balanced using randomly permuted blocks and stratified by site and age groups. The randomization statisticians will use the SAS software to generate a random list imported into the IWRS system.
At enrollment, researchers will log in to this system and enter the information of the volunteers to obtain random numbers. Before each dose, an authorized pharmacist will log in to the IWRS system and get the vaccine number. In case of an emergency, the leading investigators will determine if unblinding of volunteers is required. If the investigators decide that unblinding is needed, they should contact the sponsor first. The date and reason indicating why the blinding was broken must be recorded in the source documentation and the Case Report Form. Remarkably, key personnel of the sponsor will be unblinded at the time of immunogenicity analysis, interim analysis, and primary analysis. Sites and volunteers will remain blinded until all volunteers have completed the study.
Number of volunteers to be randomized (samples size)
The expected rate of infection with SARS-CoV-2 in children and adolescents is 1% in the placebo arm. An interim analysis is planned if 47 confirmed COVID-19 cases are observed. To evaluate 50% vaccine efficacy, a total of 93 confirmed COVID-19 cases and at least 12,593 volunteers are required to achieve 95% power with a two-sided significance level of 5%. Considering a 10% loss of follow-up, approximately 14,000 volunteers will be needed to recruit in total and 7,000 volunteers for each arm in all participating countries. If required, recruitment of volunteers may be modified if the Data Safety Monitoring Committee found it fitting upon publishing the interim unblinded analysis.
Samples acquisition
Blood and saliva samples will be obtained at times indicated in Table 1 and will only be used for scientific research. Each sample will be labeled with a code to ensure blinding. The sponsor may store the remaining samples after performing assays outlined in the protocol. Unless local regulations or ethical requirements indicate a time limitation, the samples will be stored for up to 5 years after the end of the study and then destroyed.
| Procedure | Date (Day or month relative to vaccine administration) | |||||||
|---|---|---|---|---|---|---|---|---|
| D-28 to D0 | D0 | D8 (±3) | D28 (±10) | D36 (±3) | D56 (±10) | D28 + 6 months (±30 days) | D28 + 12 months (±30 days) | |
| Screening, preliminary notification, and volunteer enrolmenta | X | |||||||
| Informed consent and assentb | X | |||||||
| Demographic information | X | |||||||
| Physical examinationc | X | |||||||
| Pregnancy examination (post-menarche girls or by the local standard of care)d | X | X | ||||||
| Inclusion/exclusion criteria screening | X | X | ||||||
| Evaluation of SARS-CoV-2 infection (IgG/IgM rapid test, rapid antigen test) | X | |||||||
| Vaccination | X | X | ||||||
| Efficacy assessments | ||||||||
| Weekly telephone/ text contacts for 8 weeks, and then every 2 weeks for monitoring of COVID-19 | Active and passive | |||||||
| Immunogenicity assessments (Sub-group) | ||||||||
| Blood collection for humoral and cellular immunogenicitye | X | X | X | X | ||||
| Saliva collection for humoral immunogenicityf,g | X | X | X | X | X | |||
| Safety assessments | ||||||||
| Participant self-recording of the safety observation on diary cards (Sub-group) | X | X | X | X | X | |||
| Monitoring of AESI/SAE, information of concomitant use of drug/vaccine | X | X | X | X | X | X | X | |
Note: a: The screening will be performed within 28 days before the study vaccination or on the day of immunization. Screening must be completed, and all eligibility criteria must be fulfilled before randomization and vaccination; b: Performed before any study-related procedure. c: Recheck clinical status before study vaccination. Physical examination, including medical history, height, and weight, vital signs, pulse, temperature, etc. Vital signs may be measured at the discretion of the investigator. Under exceptional circumstances such as high altitude, the investigator should assess baseline respiratory rate and other vital signs, as appropriate. Body temperature will be measured preferably via oral or axillary temperature or following the local standard of care; d: For participants of childbearing potential only; e: 3.0 mL of blood samples are collected for humoral immunity. 8.0 to 20 mL of blood samples are collected for cellular immunity; f: At least 2.0 mL of saliva samples are collected for humoral immunity; g: Saliva samples will also be collected on D42 (± 10), D120 (± 30), D300 (± 30) at the home of the volunteers, without a visit to the study center.
Table 1: Schedule of activities for the PedCoronaVac03CL study in Chile.
Volunteers may request their samples, if still identifiable, to be destroyed at any time; however, any data already collected from those samples will still be used for this research.
The current COVID-19 pandemic has profoundly impacted our lives, changing how we interact with each other and our mobility [24,25]. SARS-CoV-2, the etiological agent of this disease, is responsible to this date of almost 5 million deaths worldwide, placing this virus among the worst pandemics of modern history [3,26]. Several studies have shown that the disease caused by this virus is more severe in adults and the elderly compared to the pediatric population [27]. Because the symptoms of the acute illness caused by SARS-CoV-2 are less severe in children and teenagers, the transmission of this virus can reach exponential rates, as it can be confused with other flu-like diseases [27,28].
Therefore, the characterization of the safety and immunogenicity of the currently approved COVID-19 vaccines in the pediatric population is urgent and mandatory to start immunization of this population.
During October and December of 2020, 552 volunteers of the pediatric population were enrolled in phase I/II studies with CoronaVac® [12]. These volunteers were immunized with either two doses of CoronaVac® (300 or 600 SU), each separated by 4 four weeks or two doses of placebo [12]. No statistical differences were found in the incidence of adverse events reported between the two doses evaluated and the placebo group [12]. Accordingly, most adverse events were mild or moderate, with pain in the vaccination site being the most frequently reported one [12]. The seroconversion of circulating neutralizing antibodies reported for both phase I/II studies was 100% with the 600 SU dose, and no neutralizing antibodies could be detected in the placebo group. Therefore, CoronaVac® was well-tolerated and safe and induced humoral responses in children and adolescents aged 3-17 years.
The safety, effectiveness, and immunogenicity of other vaccines approved by the WHO for emergency use are also being evaluated in pediatric populations [29–31]. A phase II/III trial is being held for the mRNA-1273 vaccine (Moderna Tx, Inc.–lipid nanoparticle mRNA), which is recruiting a total of 13,275 children aged between 6 months and 12 years [29]. This placebo-controlled study will be divided into two parts, considering dose-escalation and age de-escalation, along with randomization and observer- blinding [29]. The estimated completion date for this study is June 12th, 2023 [29]. The BNT162b2 vaccine (Pfizer-BioNTech SE-lipid nanoparticle mRNA) is also being evaluated in Phase I/II/III trials, with 7,922 volunteers aged between 6 months and 16 years being recruited [30]. This trial will also be placebo-controlled and observer-blinded, but no randomization will be considered [30]. The expected completion date for this trial is June 18th, 2024 [30]. A phase II trial with the Ad26.COV2.S vaccine (Janssen Vaccines and Prevention B.V. – recombinant adenovirus) recruited teenagers between 12 and 17 years to evaluate its safety and immunogenicity [31]. This study is placebo-controlled, randomized, and double- blind [31]. Recruitment for this study is finished, and the estimated completion date is March 28th, 2022 [31]. The number of studies being held to evaluate the safety and the immune response elicited by these vaccines demonstrate the relevance of the immunization of pediatric populations. It is important to note that the aims of these studies, like the one reported in this article, are being modified as approval for emergency use of these vaccines is fulfilled in different countries [32-37].
The administration of COVID-19 vaccines to children is relevant as it will help to limit the spread of the virus and, due to the long-term consequences described after this infection, control the impact this disease has on developing immune systems long- COVID is the term used to refer to different symptoms described by some people that do not achieve a full recovery from this disease. The symptoms of this syndrome vary among reports, but they usually consider neurological ones such as headache, cognitive difficulties, anxiety, and depression, and respiratory and muscular ones, such as shortness of breath, chest pain, myalgia, and fatigue. Although most cases related to long-COVID are reported in adults, recent analyses suggest that children may also suffer from this syndrome. The terms PIMS-TS (pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2) and Multisystem Inflammatory Syndrome in Children (MIS-C) were first used early in 2020 after several reports described a series of severe cases of exacerbated systemic inflammatory responses in children, following COVID-19 resolution. This inflammatory syndrome led to intensive care unit admission of children suffering it. The symptoms are usually fever, rash, and conjunctivitis, as seen in other inflammatory syndromes such as staphylococcal toxic shock or sepsis. Besides these, more severe symptoms have been described, such as myocarditis, aneurysm, hypotension, and shock. Considering the severity of PIMS-TS and probable Long-COVID, vaccination of children and teenagers is urgent to prevent these and other possible side effects not reported yet due to SARS-CoV-2 infection.
This study was registered in Clinicaltrials.gov on August 5th, 2021. The trial identifier is NCT04992260.
The current complete protocol of this trial is available as a Supplementary File, including other relevant information such as other statistical analyses, information on Data Monitoring, other details on ethics and protocol amendments, and thorough inclusion and exclusion criteria.
Recruitment started in Chile on September 10th, 2021. Recruitment is expected to end by January 2022. The current Chilean Annex to Master protocol version 3.0 was approved on September 23rd, 2021.
The Complete Study Protocol can be found as a supplementary file.
The authors thank all the clinical teams and volunteers willing to participate in this study.
• Original trial conception was done by Qiangian Xin, Weining Meng, Xing Meng, Gang Zeng, Alexis M Kalergis
• Trial design was done by Qiangian Xin, Sanet Aspinall, Weining Meng, Xing Meng, Gang Zeng, Alexis M Kalergis
• Study protocol writing was done by Qiangian Xin, Sanet Aspinall, Weining Meng, Xing Meng, Gang Zeng
• Writing-original draft was done by Nicolás MS Gálvez, Alexis M Kalergis
• Writing-review and editing was done by Nicolás MS Gálvez, Katia Abarca, María J Álvarez-Figueroa, Susan M Bueno, José V González- Aramundiz, Nicole Le Corre, Weining Meng, Cecilia Perret, Jorge A Soto, Gang Zeng, Alexis M Kalergis
Contribution to the protocol was done by all authors reviewed and approved the final version of the protocol.
This work was supported by: The Ministry of Health, Government of Chile supported the funding of the CoronaVac03CL Study; The Confederation of Production and Commerce (CPC), Chile, supported the funding of the CoronaVac03CL Study; The Millennium Institute on Immunology and Immunotherapy, ANID-Millennium Science Initiative Program ICN09_016 (former P09/016-F) supports SMB, KA, PAG, and AMK; The Innovation Fund for Competitiveness FIC-R 2017 (BIP Code: 30488811-0) supports SMB, PAG, and AMK; SINOVAC Life Science Co contributed to this study with the investigational vaccine and placebo, and experimental reagents.
All analyzed and raw data (masked to protect the information of volunteers) will be available upon reasonable request to the corresponding authors through email after the publication of the articles containing such information. A signed data access agreement will be requested to share the data. The study protocol is also available online and included as an annex to this article.
This study was reviewed by the Scientific Ethics Committee Health Sciences UC, Protocol ID #210616012 and the Public Health Institute, Ministry of Health, Chile, approval #20674/21. The last version of this protocol and the informed consent documents for this study were approved by the Scientific Ethics Committee Health Sciences UC, approval #012321 and the Public Health Institute, Ministry of Health, Chile, approval #25820/21.
All authors approve the publication of this article.
The authors declare no competing conflict of interests.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Gálvez NMS, Xin Q, Abarca K, Álvarez-Figueroa MJ, Aspinall S, Bueno SM, et al. (2022) A Randomized Placebo-controlled Trial to Evaluate the Efficacy, Immunogenicity and Safety of an Inactivated COVID-19 Vaccine (CoronaVac®) in Children and Adolescents. J Clin Trials. 12:497.
Received: 11-May-2022, Manuscript No. JCTR-22-16854; Editor assigned: 13-May-2022, Pre QC No. JCTR-22-16854 (PQ); Reviewed: 27-May-2022, QC No. JCTR-22-16854; Revised: 03-Jun-2022, Manuscript No. JCTR-22-16854 (R); Published: 10-Jun-2022 , DOI: 10.35248/2167-0870.22.12.497
Copyright: © 2022 Gálvez NMS, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.