
Journal of Clinical Chemistry and Laboratory Medicine
Open Access
ISSN: 2736-6588

ISSN: 2736-6588
Research Article - (2024)Volume 7, Issue 4
Background: Sports anaemia is a condition associated with high levels of haemolysis after intensive exercise such as ultra-endurance rowing competitions. Studies have shown that large or continuous muscle contractions can increase the fragility of Red Blood Cells (RBCs), which can lead to intracapillary mechanical haemolysis. Toxicity from haemolysis is caused by the release of cell free Haemoglobin (cfHb), haem and iron from the lysed RBCs. Early detection of sports anaemia can prevent toxicity by facilitating earlier intervention of preventative treatments. However, there are currently no rapid, low-cost and easy-to-use sensors to detect sports anaemia.
Methods: An electrochemical sensor was developed to detect cfHb in urine which is a good biomarker for haemolysis. The sensor utilises low-cost carbon black and screen-printed electrodes. The pseudo-peroxidase activity of cfHb was used as the sensing mechanism and dried-on meta-Chloroperoxybenzioc Acid (m-CPBA) was used as the reaction oxidant. The chronoamperometric response was characterised and calibrated with Hb spiked urine before evaluating with four ultra-endurance rowers (The Enginoars) during a 37-day cross-Atlantic rowing expedition-the Talisker Whiskey Atlantic Challenge.
Results: The Limit-of-Detection (LoD) of the sensor was determined as 2.2 μM and the 5 μM recovery was 110%. The intercept was -0.28 ± 0.1 μA and the slope was -0.18 ± 0.02 μA μM-1. The mean cfHb concentration of the four rowers was 2.40, 1.56, 2.29 and 3.69 μM. The max cfHb concentration of the four rowers was 11.94, 3.77, 16.73 and 11.91 μM.
Conclusion: The study provided proof-of-principal for the sensor in ultra-endurance competitions. It showed that while there were several haemolysis spikes during the competition, cfHb levels returned to normal within 1 to 2 days.
Sports anaemia; Haemolysis; Electrochemical sensor; Urine biomarker; Sensor calibration
Sports anaemia, also known as exercise induced haemolysis, is a condition associated with high levels of haemolysis after physical activities such as running, swimming, rowing and cycling [1]. Haemolysis is the rupture of RBCs and the release of red cell contents into blood circulation [2]. Toxicity from haemolysis is caused by the release of cfHb, haem and iron from the lysed RBCs which can activate innate immune responses and inflammatory pathways [3]. This causes damage to cells and organs, particularly the kidney as this is the normal route for expulsion of excess cfHb from the circulation [4]. Haemolysis is also characteristic of several inherited, acquired and iatrogenic conditions including Sickle Cell Disease (SCD), malaria, thalassemia, Paroxysmal Nocturnal Haemoglobinuria (PNH), atypical Haemolytic Uraemic Syndrome (aHUS), COVID-19 and cardiovascular surgery, as well as exercise-induced damage [5].
In runners, sports anaemia is sometimes referred to as foot strike haemolysis as it has been hypothesised that the high levels of haemolysis are caused by plantar mechanical trauma from repeated impact with hard running surfaces [6]. This theory is supported by studies by Davidson et al., where they reported reduced levels of the cfHb scavenging protein Haptoglobin (Hp) in runners directly after marathons and long-distance runs [7,8]. However, the foot strike haemolysis theory does not explain the phenomenon in non-hard floor runners, swimmers, rowers or cyclists, leading to alternative theories being proposed [9]. Intracapillary mechanical haemolysis due to large or continuous muscle contractions is the most likely explanation. Studies have shown that intensive training can increase the fragility of RBCs [10]. Dehydration and acidosis post exhaustive exercise has been shown to cause electrolyte changes and increased plasma osmolarity/viscosity which promotes RBC damage [11]. The intensity of hypoglycaemia and increase in body temperature associated with strenuous exercise has also been shown to affect the osmotic resistance of RBCs [12,13]. Furthermore, inflammatory activation during strenuous exercise has been linked to changes in the RBC composition and increased fragility [14]. Moreover, foot strike haemolysis is likely less relevant than it was 20-30 years ago due to improvements in running shoe construction, which has significantly reduced plantar mechanical trauma [15]. It is likely that sports anaemia is caused by a variety of mechanisms including intracapillary mechanical haemolysis.
Acute Kidney Injury (AKI) and Chronic Kidney Disease (CKD) are the most common kidney injuries associated with haemolysis [16]. Kidney injury is so prevalent with haemolysis because the kidneys are the main route for cfHb clearance once Hp protective removal strategies have become overwhelmed and are therefore susceptible to dysfunction from cfHb accumulation [17]. Haemoglobinuria is the presence of cfHb in urine and is a common feature of kidney injury caused by haemolysis [18]. It is suggested that cfHb materialises in the urine once the resorptive ability of Hp for cfHb is exceeded [19]. Therefore, it is reasonable to suggest that cfHb in urine can be considered an excellent biomarker for haemolysis and by extension, an effective predictor for kidney diseases.
There are several commonly used clinical tests for haemolysis, including direct measurements of RBC survival using chromium-51 labelling, serum Hp and Hb assays and measuring reticulocyte count [20-22]. The International Council for Standardisation in Haematology (ICSH) recommends the quantification of cfHb in serum after Hp saturation using the cyanmethemoglobin method as a standard, but haemoglobinazide and oxyhaemoglobin detection methods are also used [23,24]. However, these techniques are not practical for monitoring sports anaemia in ultra-endurance competitions as they are time consuming, laborious and cannot be performed in the field as they require laboratory equipment [25]. Furthermore, while several potentially Point-of-Care (PoC) methods have been described for the detection of cfHb, these are usually optical sensors that require a variety of costly nanomaterials [26-31].
Within this manuscript we describe a PoC urinary cfHb sensor for diagnosing haemolysis in ultra-endurance athletes. The sensing method is electrochemical which has benefits over optical sensors as it enables rapid or real-time analysis, is highly accurate and reproducible, has a low LoD, is low-cost and is easy-to-use. The sensor uses highly scalable screen-printed carbon electrodes which make up the working, counter and reference electrodes. The working electrode is modified with low-cost carbon black which is highly conductive and has a unique surface architecture suited to the adsorption of cfHb. The signal is then generated by the peroxidase activity of cfHb. Hb is considered a pseudo-peroxidase which mean that under certain circumstances it displays peroxidase-like activity [32-36]. In the sensor, cfHb catalyses the reduction of hydrogen peroxide or other oxidant to generate a two-electron deficit at the electrode surface which is then made good by the electron flow from the amperometric measurement process [37]. The solid oxidant was dried onto the working electrode, so no pre-analytical mixing steps were necessary and this is highly desirable for a PoC sensor.
The sensor was characterised in the laboratory with Phosphate Buffered Saline (PBS) and using hydrogen peroxide as the oxidant before evaluating in urine with hydrogen peroxide and the dried-on solid oxidant. The sensors were then used with portable Amulet potentiostats by a team of ultra-endurance rowers during the Talisker Whiskey Atlantic Challenge where they conducted measurements onboard the Enginoars Atlantic rowing boat over 37 days. The aims of the study were to provide proof-of-principal for the sensor’s functionality in the harsh environment of the Atlantic Ocean and to determine the extent of haemolysis that the rowers experienced.
Mesoporous carbon nanopowder <500 nm particle size (from dynamic light scattering measurements), Polyethylene Imine (PEI), m-CPBA, hydrogen peroxide (H2O2, 30% w/w in H2O), phosphate buffer, Potassium Chloride (KCl), Sodium Chloride (NaCl), ethanol (absolute) and human Hb were purchased from Merck Ltd. Screen-printable inks were purchased from sun chemical. Various concentrations of Hb were prepared with deionized water obtained from a millipore Simplicity water purification system. Human urine was donated by volunteers from Imperial College London with informed consent.
Sensor fabrication
The Screen-Printed Carbon Electrodes (SPCEs) were fabricated by OG Carbon Ltd in the following manner. Carbon paste, Ag, AgCl paste and insulation ink were screen-printed onto 250 μm Polyethylene Terephthalate (PET) sheets in that order. A 30 min curing step at 60°C was included after each layer was printed. The individual SPCEs were separated and trimmed with a guillotine prior to use.
Carbon black ink was prepared by mixing 180 mg mesoporous carbon nanopowder <500 nm particle size and 45 mg Polyethylenimine (PEI) in 25 mL deionized water. The solution was dispersed by sonication using a Fisherbrand™ probe sonicator for 60 min. The solution was then mixed with a vortex mixer prior to use to ensure it was fully dispersed. 5 μL of the ink was dropcast onto the working electrode and cured at 60°C for 60 min.
24 mg/mL m-CPBA in ethanol was prepared and was dried onto the sensor by pipetting 0.3 μL of the solution onto the carbon black modified working electrode. The electrodes were then cured at 60°C for 10 min.
The surface morphology of the bare carbon working electrode, silver/silver chloride reference electrode and dropcast carbon black working electrode was examined with a Scanning Electron Microscope (SEM). The electrodes were coated with chromium and mounted onto stubs for examination. The SEM used was an EVO 15 SEM/EDX, provided by the department of Earth Science and Engineering in the Royal School of Mines at Imperial College London.
Electrochemical laboratory measurements
A Gamry reference 3000™ potentiostat was used for all electrochemical measurements. Chronoamperometry was conducted at -0.2 V for 70 s. Several conditions were evaluated with this technique including carbon black electrodes using 100 μL Hb spiked PBS containing 10 mM H2O2, carbon black electrodes modified with m-CPBA using 100 μL Hb spiked urine.
A sensor stability profile was conducted over the course of several weeks to evaluate the deterioration of the signal overtime. Carbon black/m-CPBA sensors were stored unpackaged at 22°C until testing with 100 μL urine.
Calibration of the sensors was achieved by plotting the Hb concentration against the amperometric response and fitting a linear regression. The slope and intercept were used for the calibration. A recovery value was obtained by measuring the sensor response of a known Hb concentration (5 μM) and comparing the calibrated sensor response to the known concentration. The LoD was calculated as the mean blank sample +2 Standard Deviation (SD).
Rowing study
The Talisker Whiskey Atlantic Challenge is a 3000-mile race made up of teams of four that row continuously in ocean rowing boats for up to 60 days across the Atlantic Ocean from La Gomera to Antigua. The rowers maintain a 2-hours-on, 2-hours-off schedule, follow a polyphasic sleep pattern and are therefore exposed to intense exercise for prolonged periods. The participants were provided with 300 foiled sensors, 4 portable meters, 2 pipettes and 300 pipette tips that were stored on the boat for the duration of the study.
The sensors provided were foiled by hand using mylar foil and sealed with an impulse heat sealer to prevent exposure to moisture and salt. The portable meters were supplied by Amulet, Inc. The Amulets were programmed with the chronoamperometry procedure. A Three-Dimensional (3D) printed connector port was developed for each Amulet to allow the sensor to be connected to the device.
Ethics approval was obtained from the Head of Department at the Molecular Sciences Research Hub (MSRH), Imperial College London and the College’s Science, Engineering and Technology Research Ethics Committee (SETREC). The study was conducted in accordance with the recommendations for physicians involved in research on human subjects adopted by the 18th World Medical Assembly, Helsinki 1964 and later revisions. Consent was obtained from each participant only after a full explanation was given and signed participant consent forms were obtained.
The data was downloaded from the Amulet devices to a smart phone through bluetooth after the race. The data was then transferred to Imperial College London servers for further analysis.
The Enginoars started the race on 12/12/2022 and finished on 18/01/2023 after spending a total of 37 days at sea. They rowed a total distance of 2666 nautical miles and reached a top speed of 10.5 knots (Figures 1 and 2).
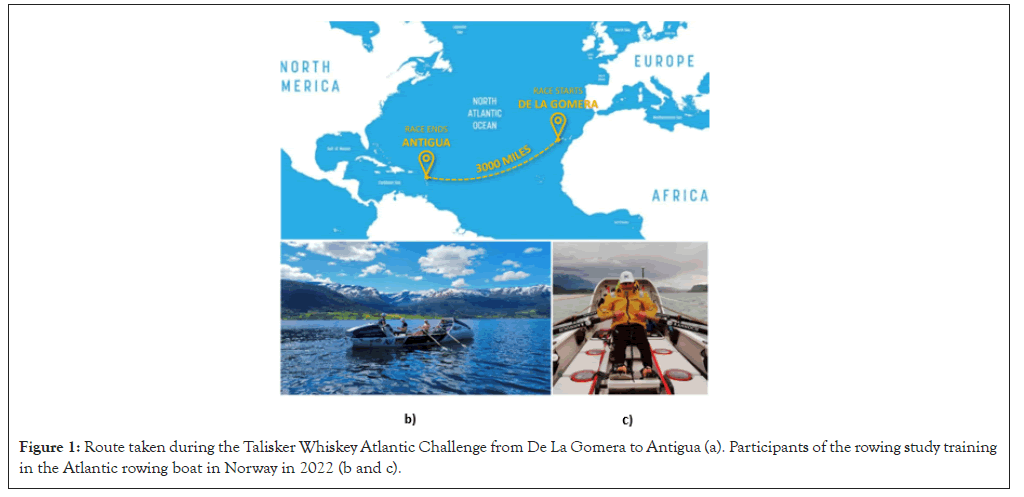
Figure 1: Route taken during the Talisker Whiskey Atlantic Challenge from De La Gomera to Antigua (a). Participants of the rowing study training in the Atlantic rowing boat in Norway in 2022 (b and c).
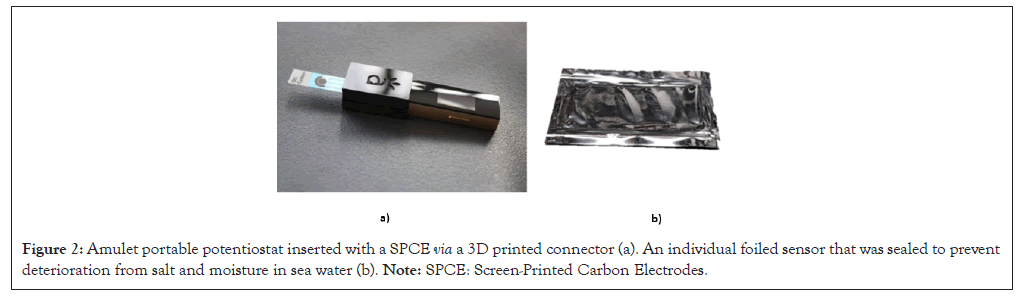
Figure 2: Amulet portable potentiostat inserted with a SPCE via a 3D printed connector (a). An individual foiled sensor that was sealed to prevent deterioration from salt and moisture in sea water (b). Note: SPCE: Screen-Printed Carbon Electrodes.
Sensor development
Chronoamperometry was used to validate the carbon black modified sensor’s amperometric response at -0.2 V using PBS with 10 mM hydrogen peroxide and a range of spiked Hb concentrations (0, 0.25, 0.75 and 1 μM). These results are shown in Figure 3 and they show that Hb’s pseudo-peroxidase activity can generate catalytic currents in the PBS medium with hydrogen peroxide as the oxidant.
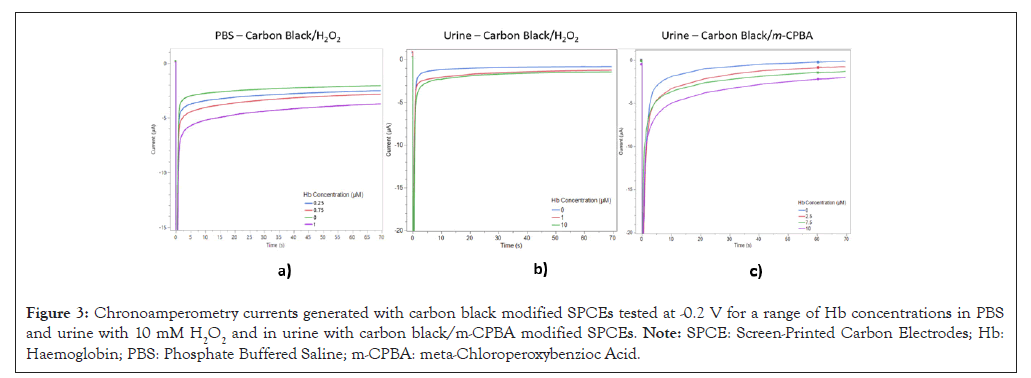
Figure 3: Chronoamperometry currents generated with carbon black modified SPCEs tested at -0.2 V for a range of Hb concentrations in PBS and urine with 10 mM H2O2 and in urine with carbon black/m-CPBA modified SPCEs. Note: SPCE: Screen-Printed Carbon Electrodes; Hb: Haemoglobin; PBS: Phosphate Buffered Saline; m-CPBA: meta-Chloroperoxybenzioc Acid.
Chronoamperometry was then used to quantify the carbon black urine sensor’s amperometric response. This was conducted at -0.2 V with 10 mM hydrogen peroxide and a range of spiked Hb concentrations (0, 1 and 10 μM). These results are shown in Figure 3 and the amperometric response forms a stable, plateaued catalytic current after 5 s.
Figure 3 also shows the amperometric response for the dried-on m-CPBA/carbon black sensors at -0.2 V using human urine and a range of spiked Hb concentrations (0, 2.5, 7.5 and 10 μM). As with the mixed in hydrogen peroxide urine sensor, the amperometric response forms a stable, plateaued catalytic current after 5 sec.
Two additional repeats with fresh carbon black modified sensors were conducted for each Hb level in urine for the carbon black/H2O2 sensor and the carbon black/m-CPBA sensor. The current generated for the plateaued (catalytic) phase of each chronoamperometric transient was calculated by taking the mean value between 5-70 sec. Linear regression was then used to fit the data and the slope and intercepts obtained from the equations were used to calibrate the two sensor types. Figure 4 shows the sensor response plots with the linear regression for the two sensor types along with the calibrated response plots. The calibrated response plots also include the 5 μM Hb concentration which was used to calculate the recovery values. The intercept, slope, recovery values, LoD and Root Mean Squared Error (RMSE) are shown in Table 1.
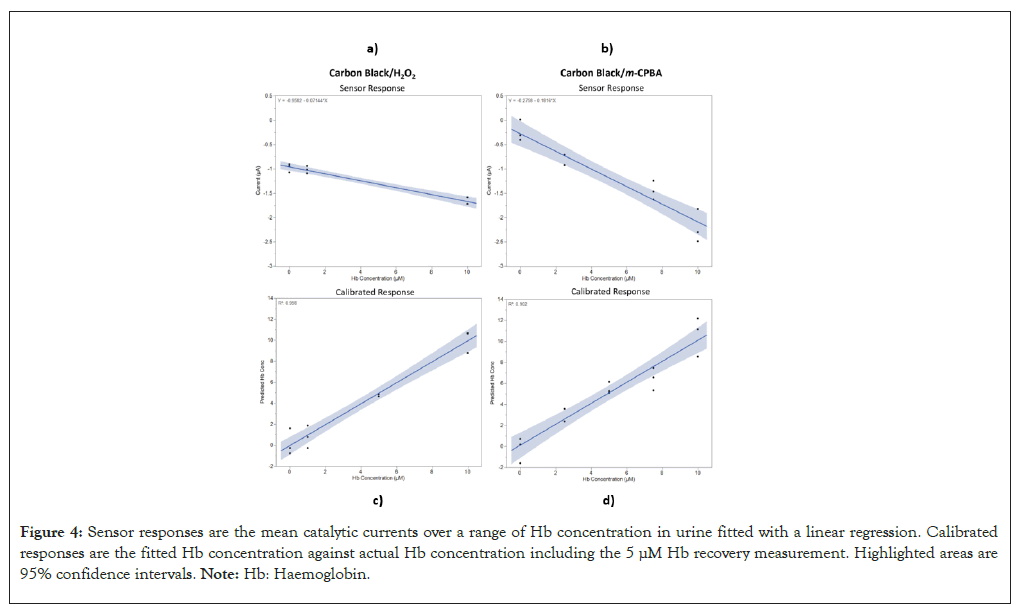
Figure 4: Sensor responses are the mean catalytic currents over a range of Hb concentration in urine fitted with a linear regression. Calibrated responses are the fitted Hb concentration against actual Hb concentration including the 5 μM Hb recovery measurement. Highlighted areas are 95% confidence intervals. Note: Hb: Haemoglobin.
| Oxidant | Intercept ± SE (μA) | Slope ± SE (μA µM-1) | 5 μM recovery (%) | LoD (μM) | RMSE (μM) |
|---|---|---|---|---|---|
| H2O2 | -0.96 ± 0.03 | -0.071 ± 0.006 | 96 | 2.7 | 0.9 |
| m-CPBA | -0.28 ± 0.1 | -0.18 ± 0.02 | 110 | 2.2 | 1.25 |
Note: H2O2: Hydrogen Peroxide; SD: Standard Deviation; SE: Standard Error; m-CPBA: meta Chloroperoxybenzioc Acid; LoD: Limit of Detection; RMSE: Root Mean Square Error.
Table 1: Intercept, slope, recoveries, LoD and RMSE calculated from calibration curves for hydrogen peroxide and m-CPBA sensor types.
A stability profile was then conducted over 22 days to evaluate the deterioration of the dried-on m-CPBA sensors. The sensors were stored in sealed petri dishes at 22°C and tested approximately every 5 days. Figure 5a shows the mean catalytic current (5-70 sec) as a percentage of the original signal.
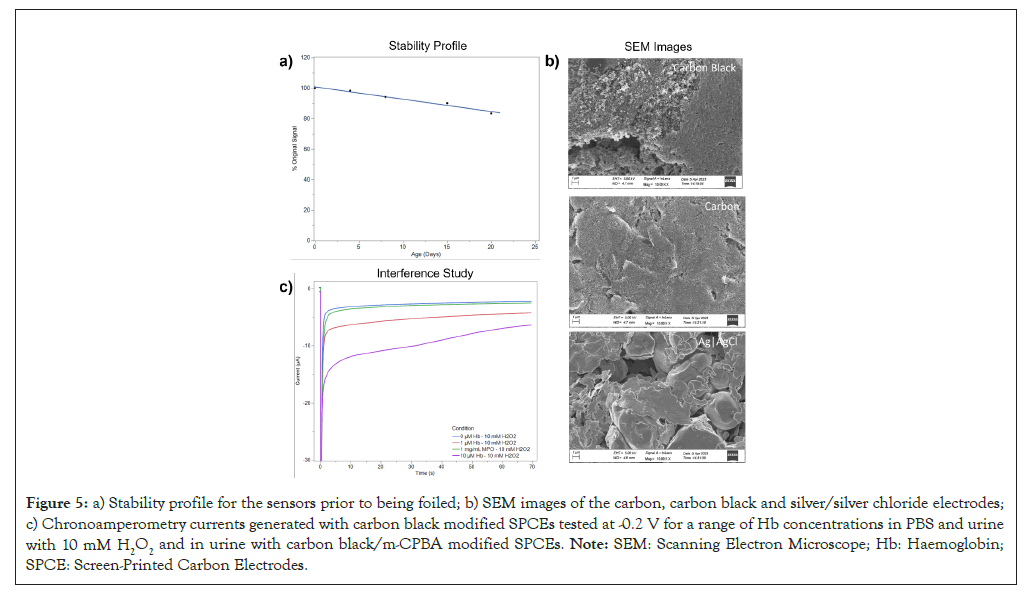
Figure 5: a) Stability profile for the sensors prior to being foiled; b) SEM images of the carbon, carbon black and silver/silver chloride electrodes; c) Chronoamperometry currents generated with carbon black modified SPCEs tested at -0.2 V for a range of Hb concentrations in PBS and urine with 10 mM H2O2 and in urine with carbon black/m-CPBA modified SPCEs. Note: SEM: Scanning Electron Microscope; Hb: Haemoglobin; SPCE: Screen-Printed Carbon Electrodes.
The surface of the bare carbon (graphite) and carbon black modified (mesoporous carbon) electrodes were inspected by Scanning Electron Microscope (SEM) to provide a qualitative assessment of the geometry and surface area. The silver/silver chloride reference electrode was also inspected. Images of these electrodes captured at 10,000 magnifications are also shown in Figure 5b.
An interference study with Myeloperoxidase (MPO) was also conducted. Like cfHb, MPO is a human peroxidase and if present in high enough concentrations it could interfere with the cfHb sensing mechanism. While studies have shown that it is usually present in very low concentrations (1 ng/mL in urine), it was nevertheless investigated as during infection it’s concentration has been reported to be up to 3.7 times higher than in sterile urine [38,39]. Chronoamperometry was used to quantify the carbon black sensor’s amperometric response at -0.2 V with 10 mM hydrogen peroxide and a range of spiked Hb concentrations (0, 1 and 10 μM) and a high MPO concentration (1 mg/mL) in PBS. The results are shown in Figure 5c.
The primary aims of the rowing study were to determine whether the carbon black/m-CPBA sensors could detect sports anaemia in ultra-endurance athletes rowing the Atlantic Ocean. A team of four rowers called the Enginoars taking part in the Talisker Whiskey Atlantic Challenge were recruited for the study as over the month-long challenge, it was likely that haemolysis would occur. The secondary aim of the study was to evaluate the performance of the sensors and Amulet portable potentiostats in the harsh environment of the Atlantic Ocean.
Initially, each of the four Amulet portable potentiostats were calibrated with dried-on carbon black/m-CPBA sensors and three levels of spiked Hb urine samples (0 μM, 5 μM and 10 μM). The results, linear regression models and equations for the Amulets are shown in Figure 6a. Linear regression was used to fit the data and the slopes and intercepts obtained were used to calibrate the Amulets. The slopes and intercepts are shown in Table 2 and the calibrated response is shown in Figure 6b.
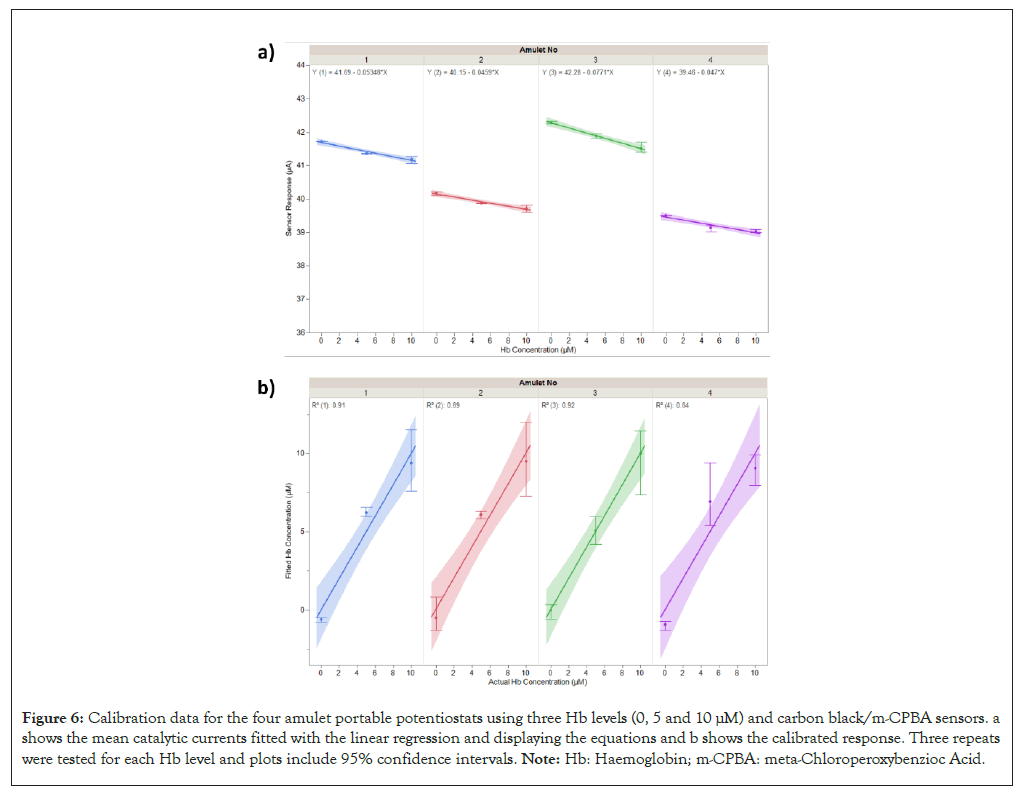
Figure 6: Calibration data for the four amulet portable potentiostats using three Hb levels (0, 5 and 10 μM) and carbon black/m-CPBA sensors. a shows the mean catalytic currents fitted with the linear regression and displaying the equations and b shows the calibrated response. Three repeats were tested for each Hb level and plots include 95% confidence intervals. Note: Hb: Haemoglobin; m-CPBA: meta-Chloroperoxybenzioc Acid.
| Rower 1 | Rower 2 | Rower 3 | Rower 4 | |
|---|---|---|---|---|
| Intercept ± SE (μA) | 41.69 ± 0.04 | 40.15 ± 0.04 | 42.28 ± 0.05 | 39.46 ± 0.05 |
| Slope ± SE (μA μM-1) | -0.053 ± 0.006 | -0.046 ± 0.006 | -0.077 ± 0.008 | -0.047 ± 0.008 |
| Mean cfHb concentration (μM) | 2.4 | 1.56 | 2.29 | 3.69 |
| SD (μM) | 2.98 | 1.53 | 3.54 | 3.36 |
| Max cfHb concentration (μM) | 11.94 | 3.77 | 16.73 | 11.91 |
| Min cfHb concentration (μM) | -0.88 | -1.4 | -0.5 | -1.03 |
Note: SD: Standard Deviation; SE: Standard Error; cfHb: cell free Haemoglobin.
Table 2: Intercept and slope obtained from the amulet calibrations and summary of the Talisker Whiskey Atlantic Challenge urinary cfHb measurements including mean cfHb concentration, SD and minimum/maximum cfHb concentration.
The field measurements were obtained over the course of the Atlantic crossing which took 37 days in total. The urinary cfHb concentration measurements as measured with the Amulet-carbon black/m-CPBA sensors for each of the four rowers over the duration of the race are shown in Figure 7a. The trendlines from start to finish for each of the athlete’s urinary cfHb concentration is shown in Figure 7b. This plot also includes R-squared values which show the goodness-of-fit for the trendlines. The mean urinary cfHb concentration, SD and minimum/maximum cfHb concentrations are presented in Table 2.
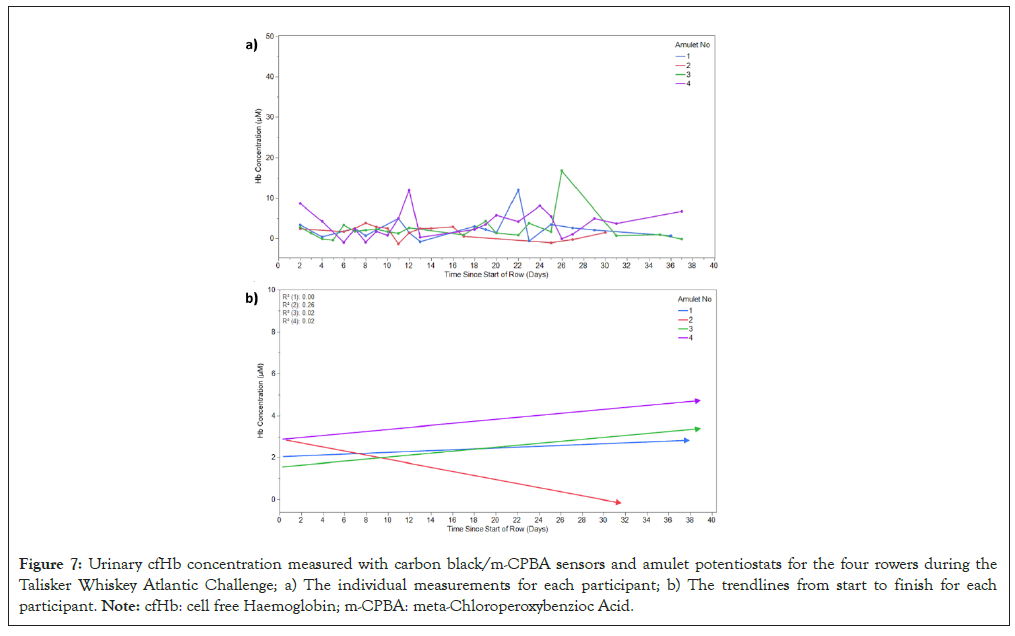
Figure 7: Urinary cfHb concentration measured with carbon black/m-CPBA sensors and amulet potentiostats for the four rowers during the Talisker Whiskey Atlantic Challenge; a) The individual measurements for each participant; b) The trendlines from start to finish for each participant. Note: cfHb: cell free Haemoglobin; m-CPBA: meta-Chloroperoxybenzioc Acid.
Sensor development
The pseudo-peroxidase activity of cfHb was successfully utilized as a method of generating a signal in the sensor. This was initially demonstrated in PBS before characterizing in urine and comparing the hydrogen peroxide and the solid dried-on oxidant m-CPBA based sensors. The intercepts for the hydrogen peroxide and m-CPBA sensors were -0.96 ± 0.03 μA and -0.28 ± 0.1 μA respectively Table 1. These intercepts are slightly larger than zero which suggests that direct reduction of the oxidants is occurring at the electrode surface, albeit to a smaller extent than reduction caused by Hb. The dried-on m-CPBA sensor intercept is also closer to zero which suggests that hydrogen peroxide is reduced at the electrode surface to a greater extent. The slope for the hydrogen peroxide and m-CPBA sensors were -0.071 ± 0.006 μA and -0.28 ± 0.1 μA μM-1 and -0.18 ± 0.02 μA μM-1 respectively Table 1. The slope is more negative for the m-CPBA sensor which suggests it has greater sensitivity than the hydrogen peroxide based sensor. The 5 μM recovery value for the hydrogen peroxide and m-CPBA based sensors were 96% and 110% respectively Table 1. While the hydrogen peroxide based sensor has a slightly superior recovery value, the 5 μM recovery value for the m-CPBA based sensors is also suitable.
The LoD for the hydrogen peroxide and m-CPBA sensors were 2.7 μM and 2.2 μM respectively Table 1. This suggests that the m-CPBA based sensor is slightly more sensitive. The LoD is also similar to those of other methods used for Hb detection. For example, the optical sensors developed by Pollard et al., and by Zhao et al., displayed LoDs of 2.56 μM and 1.55 μM respectively [26,40]. The RMSE for the hydrogen peroxide and m-CPBA sensors were 0.90 μM and 1.25 μM respectively Table 1. This is likely because the dried-on m-CPBA pipetting method is less precise than the mixed-in hydrogen peroxide method. Although the two sensor types are compared within this study, it is clear that the dried-on m-CPBA sensor is superior as it has a scalable manufacturing method and eliminates the requirement for a pre-analytical mixing step.
The stability profile in Figure 5a shows that the m-CPBA based sensors deteriorate by 15% after 20 days. Although this is a relatively minor deterioration, it highlights the requirement for the sensors to be individually foiled to prevent the deterioration. This was conducted for the rowing study and it is clear from the results in Figure 5a that the foiling of the sensors prevents their deterioration.
The SEM images in Figure 5b show that the carbon black (mesoporous carbon) modified electrode has several defect sites, which makes the surface area very large compared to the bare carbon (graphite) electrode which is relatively uniform. The large surface area of carbon black is well studied and confirms the SEM experiment’s findings, with some studies suggesting it can be as high as 1270 m2/g [41]. The large surface area of the carbon black electrode is apt for the adsorption of Hb and as such, these results confirm the material’s suitability as a working electrode material for the haemolysis sensor. The silver/silver chloride reference electrode was also inspected by SEM Figure 5b. The image captured shows that it consists of two components, which are flakes (silver) and bulbed mounds (silver chloride), which is consistent with other silver/silver chloride microscopy studies [42].
The interference study in Figure 5c shows that the signal generated in response to a large concentration of the potential interferant MPO (1 mg/mL) is not significantly larger than the baseline signal. This is highly encouraging as it is unlikely that the concentration of MPO in urine would ever be high enough to interfere with the cfHb response.
It is also worth noting that although we report here the characterisation of sensors produced by pipetting m-CPBA onto the surface of the working electrode, alternative manufacturing methods were also evaluated. These included a screen-printing method of printing an ink consisting of m-CPBA and carbon black with PEI directly onto the graphite working electrode to assess the viability of a potentially more scalable manufacturing method. We also evaluated the use of magnesium Monoperoxyphthalate (MMPP) as the solid oxidant as it is considered less hazardous to handle than m-CPBA. These different sensor types performed similar to each other in the laboratory, but only the m-CPBA sensor is reported here as it was the only one used for the rowing study.
It is clear from Figure 7a that the four rowers experienced quite different urinary cfHb concentration/time profiles from each other-which is not surprising as they all had different rowing schedules (i.e. they rowed in shifts). The mean urinary cfHb concentration values of the four rowers were 2.40 (SD=2.98) μM, 1.56 (SD=1.53) μM, 2.29 (SD=3.54) μM and 3.69 (SD=3.36) μM, respectively Table 2 which suggests that haemolysis was certainly experienced during the row. Three of the four rowers also experienced ‘spikes’ of high cfHb concentrations (i.e. 11.94 μM, 3.77 μM, 16.73 μM and 11.91 µM, respectively). These data suggest that the severity of haemolysis experienced during the Atlantic crossing was quite varied with rowers 1, 3 and 4 having the worst incidences. The minimum cfHb concentrations measured for all four rowers are within error 0 μM (i.e. -0.88 μM SD=2.98, -1.40 μM SD=1.53, -0.50 μM SD=3.54 and -1.03 μM SD=3.36). However, the large difference between the minimum and maximum cfHb concentrations suggests that the rowers rapidly recovered after the episodes of severe haemolysis. It is also conceivable that high levels of dehydration experienced by the rowers during the race also contributed to the degree of variation in urinary cfHb concentrations [43].
Figure 7b shows the trendlines from start to finish for the four rowers. These trendlines suggest that their urinary cfHb concentrations were higher at the end of the row for all rowers apart from rower 2 who’s urinary cfHb levels were lower at the end. This may suggest a moderate cumulative increase in haemolysis over the course of the race, although the R-squared values for these trendlines are low; further studies would be required to substantiate.
The primary aim of this study was to establish whether haemolysis occurred during the Talisker Whiskey Atlantic challenge. While the study was somewhat limited in the number of participants, it suggests that there were some periods of the race during which significant haemolysis occurred, but that the rowers recovered rapidly following these. The secondary aim of the study was to demonstrate the carbon black/m-CPBA sensors and Amulet portable potentiostats worked in the harsh environment of the Atlantic Ocean. The study has demonstrated that this is the case and has clearly shown that the foiled sensors can be used in such a hostile environment as the Atlantic. This further validates the sensor’s suitability as a PoC device.
In conclusion the findings of this study provide proof of principal for a sports anaemia sensor for use with ultra-endurance athletes. The performance of the non-invasive device has been demonstrated under harsh, real-world ‘field’ conditions. An acceptable LoD was obtained for the sensor that is comparable to other cfHb sensors reported in the literature. The electrochemical sensor utilized low-cost carbon black to ensure it was affordable, which is a fundamental requirement for sports devices. The use of a ‘built in’ oxidant for the Hb reaction ensured there were no additional pre-analytical steps which further validates its potential as a sports device.
A possible limitation of the method described is that the sensor was not developed for use with whole blood. However, while blood is considered a highly effective and popular test medium (mainly due to the precedent set by glucose test strips in diabetes management), urine does have some advantages, the most notable of which is that sample collection is pain-free. Whole blood sensors usually require a finger-prick with a lancet to draw a sample (or venepuncture to produce a vial of blood). Urine sensors can avoid this painful pre-analytical step by allowing the user to urinate directly onto the sensor (like a pregnancy test); this additionally limits the opportunity for adventitious infection. Moreover, the sensor’s specificity arises from the pseudo-peroxidase activity of Hb. While it is highly unlikely that there are any other peroxidases in urine at high enough concentrations to be considered an interferant, there is a greater possibility in whole blood.
While this Atlantic rowing study demonstrated that the sensor could be used as a sport science diagnostics device, it would be beneficial to increase the number of participants for future studies. It is also recommended that the sensor be additionally evaluated with athletes in other sports, such as ultra-marathon runners, as this may make it easier to recruit participants. Ultra-marathon running is also traditionally associated with very high levels of foot-strike haemolysis and may be an interesting application of the developed sensor given the high number of such athletes worldwide.
The authors would like to thank the Enginoars for their efforts and Amulet, Inc for providing the Amulets; www.amulet-inc.com
This study was provided by The Institute of Chemical Biology (ICB) Engineering and Physical Sciences Research Council (EPSRC) Center for Doctoral Training (CDT) and Roche Diagnostics International Ltd.
This study was approved by the Head of Department at the Molecular Sciences Research Hub (MSRH), Imperial College London and the Science, Engineering and Technology Research Ethics Committee (SETREC). It was performed in accordance with the ethical standards in the Declaration of Helsinki.
OTG designed and performed the experiments and analysed the data, OTG, ACS, AEGC and GQ wrote the paper, ACS, AEGC and GQ supervised the project.
Data will be made available on reasonable request.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Griffiths OT, Spivey AC, Quinlan G, Cass AE (2024). A Rapid Urine Sensor for Detection of Sports Anaemia in Ultra-Endurance Athletes. J Clin Chem Lab Med. 7:296.
Received: 29-Nov-2024, Manuscript No. JCCLM-24-35532; Editor assigned: 02-Dec-2024, Pre QC No. JCCLM-24-35532 (PQ); Reviewed: 16-Dec-2024, QC No. JCCLM-24-35532; Revised: 23-Dec-2024, Manuscript No. JCCLM-24-35532 (R); Published: 30-Dec-2024 , DOI: 10.35248/2736- 6588.24.7.296
Copyright: © 2024 Griffiths OT, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.