Journal of Agricultural Science and Food Research
Open Access
ISSN: 2593-9173
ISSN: 2593-9173
Research - (2019)Volume 10, Issue 4
This review has been checked on in 2018 at Pawe Agricultural Research Center to analyze or to evaluate what reproducing activities those are really not regular in Ethiopia, direct for rust ailment opposition. The survey was explored by assessing various diaries which have been composed for Asian Soybean Rust that is getting to be normal and cut off even in Ethiopia. Soybean rust brought about by P. pachyrhizi likewise called Asian soybean rust (ASR). Hot and damp condition is a perfect condition that can cause soybean rust infection which prompts decreased photosynthetic region on the leaves and untimely defoliation, favors illness occurrence. Rearing systems for opposition are progressively appropriate to manageable horticulture, lessens the requirement for synthetic applications and subsequently, natural harm. Diverse reproducing strategies has been applying to create opposition assortment and to control Asian Soybean Rust. Among those, screening or recognize germplasms having obstruction quality to fuse it into rust defenseless genotypes, create opposition quality trough hybridization, Resistance quality pyramiding, wide hybridization and quality quieting are the significant techniques of reproducing for advancement imperviousness to rust soybean material. So, I prescribe to that those hereditary designing based rearing systems ought to be rehearsed and acclimated Ethiopia.
Soybean; Rust; Resistance
Soybean (Glycine max L. Merrill) is one of the most significant and flexible yields around the world. Its high caliber in protein content (40%), oil content (20%), and various bioactive variables make soybean an exceptionally alluring yield with a capability of improving weight control plans of a great many individuals [1]. In addition, in cultivating frameworks, the yield is referred to improve soil properties, for example, richness through nitrogen obsession and upgrading dampness maintenance. The blend of improved soil properties and the capacity to break life cycles of bugs and infections makes soybean a perfect harvest in oat pivot programs [2]. Notwithstanding the significance of soybean, various biotic factors particularly foliar ailments decrease yields and along these lines rancher pay [1]. Soybean rust (Phakospora spp) is the most significant foliar infection in soybean creation that causes yield misfortunes of up to 80% in unprotected fields [3]. Two commit Phakopsora species; P. meibomiae (Authur) and P. pachyrhizi (Sydow and Sydow) cause soybean rust, however the last is progressively forceful and monetarily significant species in most soybean creating territories [4]. Soybean rust brought about by P. pachyrhizialso called Asian soybean rust (ASR) is a moderately new malady in Africa having been accounted for in the late 1990's [5]. In any case, its fast spread combined with the capability of causing extreme yield misfortunes makes it a significant illness of soybean. Soybean rust moved from Asia through air-borne urediniospores from India to Central Africa, at that point from Africa to South America leaving a trail of decimation for soybean cultivators [6]. Yield misfortunes going from 10 to 90% have been accounted for over the globe [7].
Ideal conditions for soybean rust proliferation
The main considerations of epidemiological significance for ASR are the general dampness of the soybean field, the quantity of stormy days, and the planting date. As a rule, conditions that advance energetic plant development and thick shelter spread advance the improvement of ASR. Infection frequency is articulated in hot, sticky situations, which results in extreme ailment assault that lessens photosynthetic territory on the leaves and prompts untimely defoliation [8]. High relative dampness of somewhere in the range of 75% and 80%, a temperature scope of 15°C to 28°C and 6 to 12 hours of dampness are required for spore germination and ailment propagation [9]. Perfect ecological conditions have caused soybean rust illness to end up endemic in most soybean developing zones in tropical Africa [10,11].
Soybean rust pathogen symptoms
Soybean rust, Phakopsora pachyrhizi (Sydow and Sydow) additionally called Asian Soybean Rust (ASR) is the significant limitation in soybean generation. Soybean rust has a firmly related animal group Phakopsora meibomiae which is a less forceful type of rust found only in Latin America [12]. These two species can't be recognized by direct perception of a swarmed field, yet just through a polymerase chain response (PCR) examine that utilizes the 20% distinction in nucleotides in the ribosomal interior deciphered locale [13]. Around the world, ASR is the best biotic risk equipped for exacting yield misfortunes of up to 80%. The pathogen assaults the yield at any formative stage causing untimely defoliation that blocks grain filling which results in harvest disappointment. Incomprehensibly, conditions that advance soybean development favors rust improvement [14]. Manifestations of soybean rust are either tan (yellowish dark colored) or red-darker relying upon the host-pathogen connection. Regularly, rust sores comprise of polygonal pustules (2-5 mm2) under the leaf (abaxial) surface with round ostioles creating urediniospores. Yield misfortunes result from diminished photosynthetic limit thus low number of filled cases per plant, units per plant, seeds per plant, weight of seed per plant and 100 seed weight [15,16]. Soybean rust shows for the most part on the leaves, petioles and some of the time on the stems of soybean plants [17]. During germination, rust pustules have been seen on the cotyledons. Soybean rust produces dark, tan to red-darker polygonal pustules (two to five mm2) on the under surface of leaves (abaxial surface), limited by the vascular groups, with urediniospores rising up out of roundabout ostioles spread by wind. Side effects can likewise show on the adaxial (upper) surface, in the propelled phases of illness improvement. Manifestations are first seen on the lower leaves as dim water-drenched sores that change to little, chlorotic territories, which increment in size and change shading to either tan or red-darker. Under reasonable conditions the illness advances upward to spread all through the shade bringing about untimely leaf yellowing and in this manner defoliation [14].
Geographical distribution of soybean rust
Asian soybean rust happens in Africa, Asia, South America and North America [18]. Soybean rust was first seen in 1903 in Japan and spread to most Australasian nations, for example, China, Taiwan, Thailand and Australia [19]. The ailment was for quite a while confined to South East Asia, Australia and India. The entry time of ASR in Africa is obscure yet recommendations are that aeronautical urediniospores spread from India to Central Africa causing the main episodes in Africa [20]. Soybean rust especially has been singled out as a noteworthy danger to soybean creation all around, and it guard and foundation in Africa has caused significant yield misfortunes [21].
Rust reaction and defense mechanisms
The plants for the most part have two primary safeguard systems against pathogens, race-explicit and race-vague obstruction. Racespecific opposition is constrained by single R qualities and for the most part less tough. Conversely, race-nonspecific obstruction is a polygenic characteristic and progressively solid [22]. There are three sorts of soybean response to contamination by P. pachyrhizi, which are related with subjective obstruction qualities (Figure 1). The principal type is the invulnerability (IM) or complete obstruction, without the nearness of conceptive structures, for example, uredinia or urediniospores. The subsequent kind is inadequate (or halfway) opposition, which prompts the advancement of red dark colored (RB) injuries. Agreeing Parlevliet and Ribeiro Do Vale et al. deficient opposition permits some development or proliferation of the pathogen in the host tissues [23,24]. At long last, the tan shaded injuries (TAN), demonstrative of helplessness [25]. Either IM or RB response are started with the early view of the pathogen destructiveness proteins by plant R proteins, concurring the old style quality for-quality obstruction hypothesis. This contradictory connection is trailed by restricted customized cell demise, called excessively touchy reaction to restrain the pathogen development. Then again, TAN response implies a perfect cooperation, without the view of the pathogen by the plant. Investigations of hostpathogen collaboration demonstrate that RB sores will in general have longer dormancy (delay) period, littler and less uredinia than the TAN sores. Despite the fact that RB type damage can likewise fluctuate in shading from light red to dim red, and now and then have bigger sores than the TAN kind. These perceptions recommend that the shade of the sores may not be a dependable marker of powerlessness or opposition [26]. Moreover, relies upon the harmfulness of the pathogen, have genotype, the communication of the host and pathogen and natural conditions [27,28].
The wide variety in the sort of response, shading and power of sporulation saw in the field can be a trouble factor in genotype portrayal ponders [29]. Other than the influence of the host genotype and levels of pathogen sporulation, the shade of the sores fluctuates with their age, particularly in the field where the contamination occasions are consistent. In crafted by Miles et al., the IM and RB responses were viewed as the exceptional types of obstruction articulation [30]. Be that as it may, halfway obstruction additionally happened in the collaboration between P. pachyrhizi and soybeans, since differences were seen between the lines with TAN sores. The variety in the quantity of uredinia is one of the parameters to be considered in the differentiation of genotypes with halfway obstruction and is contrarily connected with yield [4]. The contradictory collaboration communicated as IM phenotype is intervened by Rpp1 quality Miles et al. while the obstruction given by different qualities is portrayed by the arrangement of RB injuries, and constrained development and sporulation [30]. Soybean assortments with halfway obstruction permit the advancement of a couple of sores and restricted sporulation during the developing season Wang and Hartman [31]. Race-vague obstruction has likewise been watched. It acts by lessening the sum and pace of rust advancement, regardless of whether the sort of disease is like that delivered in profoundly powerless assortments [26]. This sort of obstruction can be effective against a large portion of the pathogen populace, being more helpful than the race specifc opposition. The troubles related with race-specific and race-vague opposition have prompted the quest for new kinds of obstruction, for example, resilience [32]. Resistance is the general capacity of soybeans to create under the pressure brought about by rust. This sort has been utilized to limit yield misfortunes related with soybean rust [33] (Figure 1).
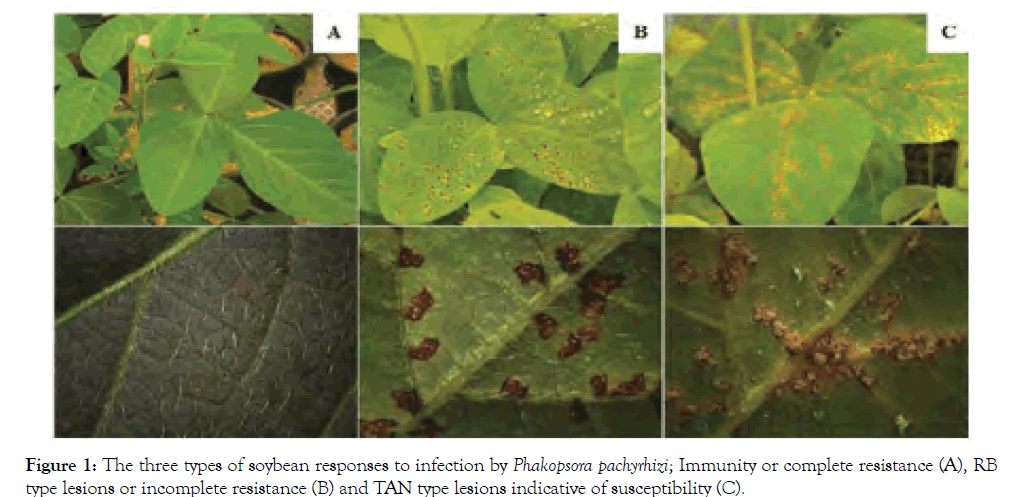
Figure 1: The three types of soybean responses to infection by Phakopsora pachyrhizi; Immunity or complete resistance (A), RB type lesions or incomplete resistance (B) and TAN type lesions indicative of susceptibility (C).
Role of breeding and strategies
Reproducing for opposition still remains the financial technique for malady control. It is more appropriate to feasible agribusiness, diminishes the requirement for substance applications and in this way, natural harm while filling in as the best long haul answer for malady the board [34].
Screening soybean germ-plasm: To control ASR, have hereditary obstruction remains the most monetarily practical, ecologically well-disposed and deliberately significant alternative for asset compelled ranchers in the creating scene. Three methodologies have been commonly used to improve assortments for protection from ASR, specifically explicit opposition, incomplete obstruction, and yield security or resistance. Screened and recognizing soybean imperviousness to rust sources has been a noteworthy target [35]. Presently, such work is being embraced by a few reproducing programs around the world, with most noticeable quality in China and the United States of America.
Different choice techniques are utilized for distinguishing proof of those descendants that have the most helpful mixes of the ideal attributes. The decision of the determination technique relies upon reproducing goal and different factors, for example, the accessible inconstancy, accessibility of horticultural machines and green house, size and ability of rearing group, and so forth [36]. The organization of explicit single qualities for opposition is in this way probably not going to be an effective system [37].
The strategies utilized for obstruction assessments are chosen dependent on unwavering quality of the outcomes and accessibility of assets (i.e., time, work, nursery space, and ability) [38]. Marker helped determination (MAS) techniques known as forward choice has been utilized adequately in soybean since the mid-1990s to prescreen reproducing populaces for basically acquired traits [36]. Be that as it may, numerous intricate qualities have not been amiable to advance determination in light of the fact that quantitative attribute loci (QTL) recognized inside one hereditary setting has not been adequately prescient of other hereditary settings [39].
Resistance (R) gene pyramiding: Quality pyramiding, which includes gathering numerous attractive qualities into a solitary genotype has been recommended as one approach to beat opposition unsteadiness given by single quality obstruction in numerous pathogens including soybean rust [42]. Fusing such numerous quality obstructions has stayed a test utilizing customary strategies because of the required broad screening utilizing quality explicit pathogen races [43].
Traditional methodologies are not in every case for all intents and purposes practical in quality pyramiding given the way that a few qualities were recognized utilizing outside races whose entrance presents calculated and phyto-sterile difficulties. In like manner, marker helped choice was the most attractive option accessible for pyramiding obstruction qualities.
Examination of soybean genotypes uncovered six predominant R qualities presenting invulnerability (no unmistakable side effects) or opposition (ruddy dark colored injuries and decreased sporulation) to explicit P. pachyrhizi confines. Those loci were alluded to as Rpp1–6 qualities [44]. In any case, Rpp qualities give obstruction solely to singular P. pachyrhizi separates (race-explicit ailment opposition). Three latent qualities to P. pachyrhizi have been recognized in the soybean genotypes PI 200456, PI 224270, and BR01-18437. These qualities are currently anticipating abuse in rearing and hereditary designing for SBR obstruction.
Creating tip top lines and assortments expects raisers to join qualities from different guardians, a procedure called quality pyramiding or stacking [45]. Pyramiding R qualities into a solitary hereditary foundation is another proposed methodology for giving soybean protection from different P. pachyrhizi disconnects [46]. The SBR safe Japanese soybean cultivar Hyuuga speaks to a characteristic case of R quality pyramiding [47]. In accordance with this discovering, soybean genotypes harboring two pyramided Rpp qualities displayed higher SBR obstruction than their predecessors containing just single R qualities [48] (Figure 2).
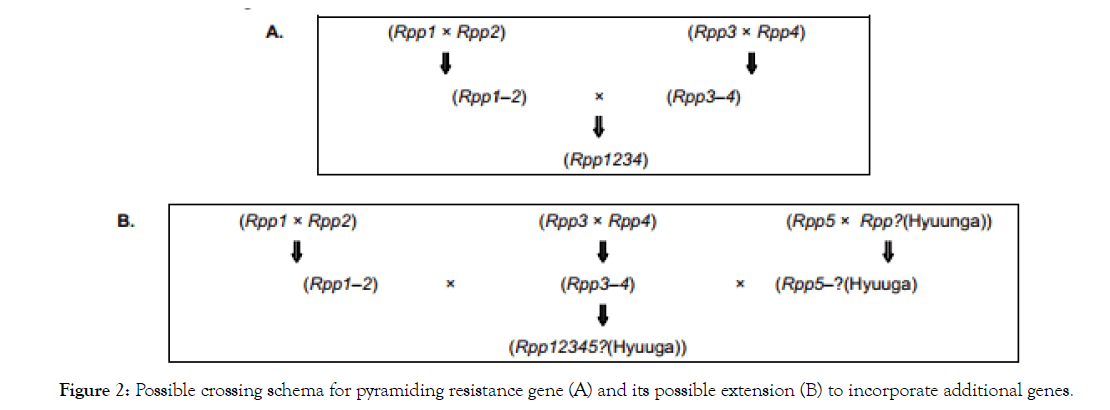
Figure 2: The three types of soybean responses to infection by Phakopsora pachyrhizi; Immunity or complete resistance (A), RB type lesions or incomplete resistance (B) and TAN type lesions indicative of susceptibility (C).
RNA interference and host-induced gene silencing: Another choice for controlling SBR is by utilizing RNAi to explicitly quiet basic P. pachyrhizi qualities. A particular RNAi strategy, gave security from infections assaulting [49]. Fruitful quieting of contagious qualities, including those of the rust parasites pucciniastriiformis, P. triticina, and P. graminis in different yields is demonstration of the tremendous capability of this methodology for battling SBR [50].
Wide hybridization: Sinclair and Hartman in 1996 announced the variety Glycine Wild to be separated into two subgenera, Glycine and Soja (Moench) F.J. Herm. The subgenus Soja incorporates the developed soybean G. max (L.) Merr [35]. what's more, the wild soybean, G. soja Siebold and Zucc. The two species are yearly, diploid with 2n=40, and hybridize promptly. Soybean develops just under development, while G. soja develops wild in China, Japan, Korea, Taiwan and Russia. G. max and G. soja structure the essential genetic supply for the developed soybean. G. soja is the wild precursor of the soybean [51]. The half and halves from intrasub generic crosses with G. max have large amounts of obstruction yet have not been abused in soybean rearing projects. Joining imperviousness to rust from the lasting species into developed soybean through wide hybridization has been generally insufficient on account of the issues related with sterility of the subsequent half and halves. There has likewise been an absence of exertion by mainstream researchers, presumably on account of case premature birth, which is a post-preparation issue [35].
Resistance genes
Safe cultivars have qualities that are powerful in the particular geographic locale where the cultivars are well on the way to be developed [51]. Soybean rearing for protection from Asian rust has been focused on subjective qualities named Rpp1, Rpp2, Rpp3 and Rpp4 since 2001 [52,53]. Other significant qualities presenting (monogenic) opposition have been recognized and presented in the rearing projects since 2004. Specific protection from P. pachyrhiziis known, and four single overwhelming qualities have been recognized as Rpp1, Rpp2, Rpp3 and Rpp4 [54-59]. The Rpp1 was portrayed as having a safe response when vaccinated with a couple separates, including India 73-1. Immunization of some rust confines on Rpp1 or different qualities delivers a safe red-dark colored sore with no or meagerly sporulation uredinia. The red dark colored injury type is viewed as a safe sore sort when contrasted and a completely vulnerable TAN sore. Single-quality opposition has not been tough, and the handiness of the wellsprings of single qualities was in viable not long after the sources were recognized [60].
Partial resistance: Utilization of single qualities to control rust may have some utility; however different choices, for example, utilizing fractional obstruction might be expected to grow "moderate rusting" cultivars. Fractional opposition, or rate lessening obstruction, is additionally known in soybean [61,62]. Lines with incomplete opposition in field assessments are appraised as respectably safe, since less sores create on plants all through the season. In nursery thinks about, have pathogen mixes that brought about RB response types would in general have longer inert periods, lower paces of increment in pustule number after some time, and littler injuries contrasted and powerless associations that brought about a TAN response type [63]. Recognizable proof and use of halfway opposition in reproducing projects has been constrained. The assessment techniques might be tedious and hard to join into rearing projects and accordingly constrained to use with cutting edge ages. These challenges, in any event to some degree, prompted the advancement of a methodology to choose genotypes with what was characterized as resistance or yield soundness in spite of being vigorously tainted with P. pachyrhizi [64,65].
Yield stability: Yield soundness, or resistance, alludes to the system of choosing genotypes with high return potential and less yield misfortune from soybean rust. Screening for yield steadiness to soybean rust was begun at the Asian Vegetable Research and Development Center, where yields from matched plots, with and without the fungicide Dithane M-45 connected at regular intervals, were contrasted for misfortunes due with rust. Highyielding genotypes with lower yield misfortune under extreme rust conditions were viewed as tolerant [65]. Rust improvement rates and gauges of rust seriousness on foliage were not associated with yield misfortune in tolerant materials.
Specific resistance: Varieties with explicit obstruction qualities produce insusceptible responses with no noticeable indications when vaccinated with explicit separates, while some produce reddark colored sores with meager uredinia To date, six race-explicit qualities have been recognized: Rpp1, Rpp2, Rpp3, Rpp4, Rpp5 and Rpp (Hyuuga). These six free opposition qualities Rpp1–5 and Rpp (Hyuuga) were distinguished in increases PI 200492, PI 230970, PI 462312, PI 459025, PI 200456 and PI 506764, individually. What's more, other obstruction qualities and their source material exist, however without explicit names, for example, in PI 398507, FT2, PI 407912, PI 424473 and UG5 [65]. The reason for distinguishing these obstruction qualities is phenotypic assessment of sickness seriousness, sore sort, sporulation degree and number of uredinia per injury.
Soybean rust brought about by P. pachyrhizi likewise called Asian soybean rust (ASR). Hot and sticky condition is a perfect condition that can cause soybean rust ailment which prompts diminished photosynthetic territory on the leaves and untimely defoliation, favors illness frequency. Distinctive reproducing strategies has been applying to create opposition assortment and to control Asian Soybean Rust. Among those, screening or recognize germplasms having opposition quality to fuse it into rust helpless genotypes, create obstruction quality trough hybridization, Resistance quality pyramiding, wide hybridization and quality quieting are the real methodologies of reproducing for improvement imperviousness to rust soybean material.
Citation: Mekonen AA (2019) A Review on Role of Breeding for Rust Disease Resistance in Soybean (Glycine max [L.] Merrill). J Agri Sci Food Res. 10:269. doi: 10.35248/2593-9173.19.10.269
Received: 17-Sep-2019 Accepted: 19-Nov-2019 Published: 26-Nov-2019
Copyright: © 2019 Mekonen AA. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.