Journal of Chromatography & Separation Techniques
Open Access
ISSN: 2157-7064
ISSN: 2157-7064
Research - (2022)Volume 13, Issue 2
An analytical method to simultaneously determine Alginic acid, Laminarin and Mannitol in seaweed extracts applied as fertilisers was developed using ultra High-Performance Liquid Chromatography (HPLC) coupled with a Refractive Index Detector (RID). A simple sample treatment for liquid and solid products is proposed involved, dilution with ultrapure water, added glycerol as Internal Standard (IS) and centrifugation step. Chromatographic analysis (25 min) was performed on a Bio-Rad Aminex HPX-87H column and a mobile phase consisting of 0.05% acetic acid solution at a flow rate of 0.5 ml/min. The method was fully validated in terms of selectivity, Limits of Detection (LOD) and Limits of Quantification (LOQ), linearity, recovery, precision and robustness. Finally, the proposed method was successfully applied to analyze the three analytes in different commercial samples. This method was demonstrated to be an innovative tool in the quality control and investigation of these analytes in seaweed fertilizers in a single run.
HPLC-RID; Biostimulant; Legislation; Ascophyllum nodosum; Ecklonia maxima
AA: Alginic Acid; GRO: Glycerol; UHPLC: Ultra High-Performance Liquid Chromatography; IS: Internal Standard; Lam: Laminarin; LOD: Limit of Detection; LOQ: Limit of Quantification; Man-ol: Mannitol; RID: Refractive Index Detector
In the last five decades, agriculture has undergone a transformation called the “Green Revolution” based on the accelerated increase in food production due to the selective crossing of species and use of fertilisers, pesticides and new irrigation techniques [1]. One consequence of greater productivity and economic profitability has been profound environmental deterioration. Agricultural practices are the main source of water pollution by nitrates, phosphates, pesticides, and the world’s loss of biodiversity [2,3]. Thus the goal of food production is to use sustainable practices that increase the production and quality of crops but have a minimal impact on the environment. These objectives are framed in what is known as sustainable agriculture [4-6].
Biostimulant products are important tools in modern agriculture and contribute to it becoming more sustainable and resilient. According to the European Biostimulants Industry Council plant biostimulants contain substance(s) and/or microorganisms whose function when applied to plants or the rhizosphere is to stimulate natural processes to enhance plant nutrient uptake, nutrient use efficiency, tolerance to abiotic stress and crop quality [7,8]. These products minimize the use of chemical products, reinforce plant defences and make them healthier and stronger when confronted with pests and diseases. Thus, waste is avoided and costs reduced for farmers. The biostimulant market size was calculated to be ca. EUR 1.45 billion in 2016, and is expected to rise to ca. EUR 2.66 billion by 2022 [9]. The European Union has recently included these products as fertilizer products in regulation CE 2019/1009, which comes into force in July 2022. It is therefore necessary to have an adequate analytical methodology for assessing whether or not a product complies with current legislation.
Seaweed extracts are some of the widely used biostimulants. It is estimated that there are close to 10,000 macroalgae species [10]. Those are subdivided into three categories based on their pigmentation. The most interesting species for use in agriculture are brown seaweeds. Some species, including Ecklonia maxima and Laminaria digitata, always grow submerged in water. Species such as Fucus sp. or Ascophyllum nodosum support periods of immersion and periods when they are exposed to the elements, in accordance with tidal cycles every 12 hours [9]. This condition of development is a phenomenon of physiological adaptation, with particular consequences in the biochemical composition of these algae and thus conferring them with important properties for use in agriculture. A. nodosum is used in the majority of commercial formulations [11]. After seaweed collection, a process to extract the active principles is carried out involving a cell disruption to release the components of interest, thus modifying the final composition of the product [12]. There are two main extraction processes that are commonly applied: Chemical extraction with acid media or potassium hydroxide and cold extraction under high pressure [13-16].
Currently, in order for a seaweed extract to be marketed in Europe, it is necessary to provide the content of Alginic Acid (AA), one of the main constituents of cell walls, and Mannitol (Man-ol), which protects plant cells from the negative effects of hydric or saline stress. For Liquid Products (LP), the minimum content established by the EU’s regulation is 1.5% (w/v) AA and 0.5% (w/v) Man-ol, while for Solid Products (SP) it is 9% (w/w) AA and 3% (w/w) Man-ol. To the authors’ knowledge, there are a few methodologies for determining the content of AA, one of them is based on a spectrophotometric method being the most used. In the case of Manol, the EU regulation proposes anion exchange chromatography to be the optimal technique, however there is no official method and almost no related studies [16, 17]. Laminarin (Lam) is a reserve polysaccharide formed by units of glycoses and is found in brown algae. Although EU legislation does not specify anything about this compound, its presence will allow discrimination of the type of algae analyzed and provide valuable information. To determine Lam, a common option is the use of Megazyme kits as enzymatic yeast beta-glucan kit based on a spectrophotometric method.
In view of the reported absence of specific analytical methods, the aim of this study was to develop a simple and robust analytical methodology to quantify AA, Lam and Man-ol simultaneously by means of HPLC-RID in seaweed extracts used as fertilizers. To the authors’ knowledge, there have been no reports about a simultaneous analysis of these compounds in seaweed extracts used as fertilizers. It was therefore decided to perform their separation using a Bio-Rad Aminex HPX-87 H analytical column. The effects of various parameters were studied, such as mobile phase composition, pH, flow rate and use of Internal Standard (IS). A further goal of this study was to undertake a full validation of the proposed method to determine AA, Lam and Man-ol in commercial products, both in their liquid and solid states. This methodology is presented as a tool for the quality control of algae products as well as to facilitate research on the effect of its application.
Chemicals and reagents
Alginic acid from brown marine algae, Laminarin from Laminaria digitata, and D-mannitol (Man-ol, >99%) and Inositol (IN, >99%) analytical standards were purchased from Sigma-Aldrich Laborchemikalien (Seelze, Germany), and glycerol (Gro, >99%) and D-(+) xylose (Xyl, >99%) were obtained from Merck KGaA (Darmstadt, Germany). Acetic acid (99.8%) was obtained from Sigma-Aldrich Chemie (Steinheim, Germany) and sulphuric acid was supplied by VWR Chemicals (Llinars del Vallès, Spain). Ultrapure water was obtained using Milipore Milli-RO plus and Milli-Q systems (Bedford, MA, USA).
A vortex mechanical mixer from Labolan S.L. (Navarra, Spain), a Hettich Rotofix 32A centrifuge from Hettichlab (Tuttlingen, Germany), a laboratory bath from J.P. Selecta S.A. (Barcelona, Spain) and a Moulinette blender device from Mandine (Boulougne, France) were employed for sample treatment. Nylon syringe filters (17 mm, 0.45 μm) were supplied by Labbox (Barcelona, Spain).
Standard solutions
Individual standard stock solutions were obtained by dissolving 10 mg in 10 ml NaOH 0.10 M for Alginic acid and ultrapure water for Laminarin, Mannitol and glycerol to a final concentration of 1000 mg/l. The intermediate solutions were prepared by diluting the stock solution in ultrapure water to obtain concentrations of 5,10,25,50,100,200 mg/l and 50 mg/l of glycerol (IS) was added in each stock solution.
Sample procurement and treatment
Samples: Several representative seaweed fertilizer samples were selected in this study according to the way in which they are marketed–Liquid Products (LP, n=12) or Solid Products (SP, n=5) –species (Ascophyllum nodosum, Ecklonia maxima or a mixture) and pH (from 3.20 to 10.8). All the samples were kindly provided by different fertilizer companies (n=15) and by Laboratorio Arbitral (Spain’s Ministry of Agriculture, Fisheries and Food; MAPA) (n=2).
Sample treatment: Briefly, 40 mg of LP or 20 mg of SP were diluted in 100 ml (LP) or 250 ml (SP) of ultrapure water. For SP is necessary a previous step based on crushed with a grind and passed through a 40 mesh screen to obtain a homogenous sample. The mixture was shaken in a vortex for 1 min and then centrifuged for 5 min at 3000 rpm. The samples were filtered through 0.45 µm nylon filter before injection into the HPLC-RID system.
HPLC-RID analysis
The analyses were performed on a 1260 Infinity HPLC system (Agilent Technologies, Waldbronn, Germany). Open LAB CDS Rev.C.01.05 v.37 software was used for system control and data acquisition. The system consisted of an online vacuum degasser, a quaternary pump, an autosampler, and a thermostated column compartment equipped with a Refractive Index Detector (RID) (model 1260 series). A Bio-Rad Aminex HPX-87 H column (300 × 7.8 mm, 9 µm) was used, protected by a guard column from Phenomenex (Torrance, CA, USA). Chromatographic conditions were set as follows: The mobile phase was a 0.05% acetic acid, the flow rate was 0.5 ml/min, the injection volume was 50 µL, and the column temperature set at 65°C. The temperature of the refractive index detector was maintained at 50°C and the signal was acquired in positive polarity mode. Compounds were identified according to their retention times by comparing them with standards. Quantification was achieved using Internal Standard (IS) calibration employing glycerol in order to reduce analysis error (n=6) and fluctuations on the signal acquired.
Method validation
Validation was performed in accordance with the Eurachem methods validation guide determining Limits of Detection (LOD) and Limits of Quantification (LOQ), as well as linearity, matrix effect, intra-day and inter-day precision, and robustness [18]. The sample treatment studies and validation were carried out using a liquid and solid product donated by MAPA. These samples are part of an inter-laboratory test involving the participation of different fertilizer analysis laboratories.
Optimization of the sample treatment
It should be noted that fertilizer companies had previously performed an extraction of the algae’s main components, already extracting these compounds of interest from the seaweed extracts, thus it was appropriate to propose sample dilution as a suitable sample treatment. To optimize sample treatment, samples donated by MAPA were selected as the reference material and was used to optimize the sample treatment.
Firstly, the amount of sample to be analyzed was considered, and after several tests (5–100 mg), 20 mg and 40 mg were selected as the maximum amount to be used for SP and LP, respectively. Dilution assays were conducted with the following solutions: Ultrapure water, 0.05% acetic acid solution and sodium hydroxide 10 mm. When hydroxide 10 mm solution was employed, AA experienced a strong broadening of its peak and was therefore discarded. In the case of 0.050% acetic acid solution and ultrapure water, similar results were obtained, in order to facilitate the sample treatment ultrapure water was selected as optimal solvent. The influence of certain extraction parameters such as volume (10–500 ml) and centrifuge time (5–15 min) was sequentially tested to select the best conditions. Optimal condition was achieved with 100 ml of water for LP and 250 mL for SP and 5 minutes of centrifugation at 3000 rpm. Following this an aliquot of 1.00 ml was filtering it through 0.45 µm Millipore cellulose membrane prior to HPLC analysis.
In order to ascertain the effectiveness of the proposed sample treatment, the method was proved by comparing the peak areas obtained from blank samples spiked at three different concentrations (QC levels), either prior to (BF samples) or following (AF samples) sample treatment. Recovery values ranged from 80% to 99% in all cases for LP and SP (Table 1), indicating that the sample treatment was appropriate and effective.
| LP | SP | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Evaluation of the sample treatment Mean (%) ± RSD (%) |
Evaluation of the matrix effect Mean (%) ± RSD (%) |
Evaluation of the sample treatment Mean (%) ± RSD (%) |
Evaluation of the matrix effect Mean (%) ± RSD (%) |
|||||||||
| QC1 | QC2 | QC3 | QC1 | QC2 | QC3 | QC1 | QC2 | QC3 | QC1 | QC2 | QC3 | |
| AA | 97 ± 3 | 92 ± 7 | 83 ± 6 | 99 ± 5 | 98 ± 3 | 97 ± 5 | 89 ± 4 | 85 ± 3 | 80 ± 8 | 91 ± 6 | 95 ± 4 | 95 ± 5 |
| Lam | 98 ± 2 | 95 ± 3 | 88 ± 2 | 100 ± 2 | 99 ± 5 | 99 ± 4 | 90 ± 5 | 88 ± 4 | 82 ± 3 | 99 ± 3 | 102 ± 3 | 101 ± 6 |
QC1: (5 mg/L); QC2: (50 mg/L); QC3: (200 mg/L)
Table 1: Evaluation of the efficiency of the sample treatment (recoveries; mean value (%) ± RSD (%)) and the matrix effect (comparison of responses; value (%) ± RSD (%)) for LP and SP
Chromatographic optimization
In previous studies, most of the separations for carbohydrates with RID in seaweed extracts were conducted using the Bio-Rad Aminex HPX-87H column and successfully produced results [19- 20]. It was therefore decided to optimize the chromatographic conditions using this column. Some experiments were conducted using different mobile phases in isocratic elution mode due to it being necessary for RID analysis to avoid variations in signal acquisition. These were ultrapure water, 4 mm sulphuric acid and 0.05% acetic acid solution. The best results in terms of resolution and peak symmetries were obtained when acids were used in the mobile phase, and both provided similar results. However, one of the goals was to develop a method in accordance with the principles of Green Chemistry, therefore sulphuric acid was discarded since it is a hazardous substance and acetic acid was selected because it is emitted by the environment and not considered toxic [21]. The ion strength of the mobile phase was evaluated by varying the acetic acid percentage between 0.010 and 1.0%. The analytes presented different behaviours: Man-ol exhibited the largest peak area with 0.050% acetic acid and decreased with higher percentages. In contrast, AA and Lam showed a maximum value with 0.010% acetic acid and slight variations at higher percentages. It should be noted that AA experienced a strong broadening of the peak at this percentage; therefore the best results for all analytes in terms of S/N and peak shape were obtained with 0.05% acetic acid in ultrapure water (Figure 1).
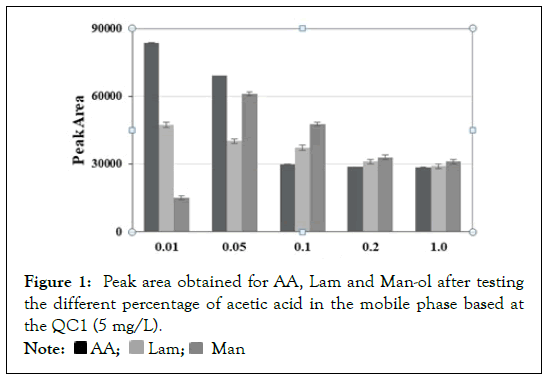
Figure 1: Peak area obtained for AA, Lam and Man-ol after testing
the different percentage of acetic acid in the mobile phase based at
the QC1 (5 mg/L).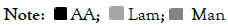
Another parameter under consideration was the impact of column temperature on the retention. This was therefore evaluated in increments of 10°C from 35°C to 65°C (maximum value recommended by the manufacturer). As shown in Figure S1, a temperature-dependent resolution of analytes was achieved. The peaks were not properly resolved at temperatures below 55°C and were resolved well with increasing column temperature. As the temperature rose, increased interactions between the carbohydrates and the surface of the stationary phase (sulfonated divinyl benzenestyrene copolymer) were observed. The optimal value in terms of resolution and peak shape was 65°C. The effect of flow rate on retention time was also studied from 0.3 to 0.6 ml/min, with higher flows providing pressures above 100 bar (not recommended by the manufacturer). As expected, the retention times decreased when the flow rate was higher. However, when operating at a flow rate of 0.6 ml/min, the pressure was at the limit of what is recommended (109 bar). Therefore, the optimal flow rate was established to be 0.5 ml/min. The possibility of enhancing the sensitivity (LOD/ LOQ) of the method by injecting larger sample volumes (5-100 µL) was considered and the results showed an increase in the Signal-to- Noise (S/N) ratio when up to 50 µL was injected, above which S/N did not significantly improve and an important peak broadening was evident. Thus, 50 µL was selected as the injection volume.
To undertake a correct quantification as recommended by most validation guides, it was decided to use an Internal Standard (IS). Different IS were studied: Inositol, Xylose, Sorbitol and Glycerol. As shown in Figure 2, Inositol, Xylose and Sorbitol peaks were not sufficiently separated from Man-ol, making them unsuitable. Glycerol (GRO) was separated completely from all target analytes, making it suitable as an internal standard for quantification of AA, Lam and Man-ol
Figure 2: HPLC-RID chromatograms for peaks of standard (AA, Lam and Man-ol) and internal standards (inositol, xylose, sorbitol and glycerol) with a time offset between signals of 10 %.
Validation of the method
Validation was carried out following the Eurachem’s Laboratory Guide to Method Validation [18]. The analytical characteristics of the method are given in Table 2.
| LP | SP | |||||
|---|---|---|---|---|---|---|
| AA | Lam | Man-ol | AA | Lam | Man-ol | |
| tR±SD | 7.05 ± 0.16 | 7.49 ± 0.05 | 12.4 ± 0.09 | 7.00 ± 0.09 | 7.47 ± 0.01 | 12.2 ± 0.1 |
| Linearity range (mg/L) | 5-200 | 5-200 | 5-200 | 5-200 | 5-200 | 5-200 |
| Slope confidence interval ± SD with IS | 7.65x10-1 ± 5.12 x10-3 | 7.73x10-1 ± 6.12 x10-3 | 7.87x10-1 ± 7.10x10-3 | 7.33x10-1 ± 4.98 x10-3 | 7.52x10-1 ± 6.01 x10-3 | 7.72x10-1 ± 6.94x10-3 |
| R2 with IS | 0.9992 | 0.9987 | 0.9998 | 0.9981 | 0.999 | 0.9991 |
| R2 without IS | 0.9899 | 0.9877 | 0.9912 | 0.9712 | 0.9823 | 0.9901 |
| LOD ± SD (mg/L) | 0.3 ± 0.08 | 0.4 ± 0.04 | 0.8 ± 0.03 | 0.6 ± 0.02 | 0.5 ± 0.09 | 0.7 ± 0.04 |
| LOQ ± SD (mg/L) | 1.1 ± 0.10 | 1.5 ± 0.046 | 2.5 ± 0.092 | 2.2 ± 0.19 | 1.8 ± 0.063 | 2.6 ± 0.032 |
Table 2: Calibration curve data (n=6), LOD and LOQ values for LP and SP.
The sensitivity of the method was evaluated experimentally in terms of LOD and LOQ as three and ten times the S/N. Low LODs and LOQs were obtained in all cases, with LODs ranging from 0.3 to 0.8 mg/l and LOQs between 1.1 and 2.6 mg/l. It should be noted that these LOQ values were much lower than the values expected in commercial products (up to 50 mg/l). In order to evaluate the suitability of Gro as an internal standard, two calibration curves were used with and without IS. When no IS was applied, linear calibration curves were obtained, but there was a significant deviation in signal intensity. When IS was applied, the linearity for all analytes increased (R2>0.99) and the deviation decreased by compensating for deviations in system performance. Therefore, to ensure the method is robust and to compensate for deviations, IS must be used. Finally, the lack of bias was confirmed by a t test and a study of the distribution of residuals. The matrix effect was calculated to assess the influence of co-extracted compounds on analytical signals. To evaluate it, a comparison was made of the responses (analyte peak area) of standard working solutions in solvent and blank seaweed extracts (AF samples) spiked at three different concentrations (QC levels). Responses at the different concentrations assayed ranged from 91% to 101% in all cases, which met the criteria of the validation guidelines (± 20% of the response from standard solution). Consequently, the matrix did not affect the signal of the analytes in seaweeds extracts and quantification could be performed with standard calibration curves. The precision of the method was evaluated by repeated sample analysis using three concentrations (QC levels: QC1=5 mg/l, QC2=50 mg/l, QC3=200 mg/l) as repeatability, intra-day experiments on the same day (n=6) and intermediate precision and inter-day experiments over three consecutive days (n=6). Precision is expressed as the percentage of relative standard deviation (% RSD), and this was always lower than 5%. These results indicated that the present method was precise and only had minor systematic errors (Table S1). Robustness of the method was evaluated by deliberate variation in chromatographic conditions such as flow rate (0.5 ± 0.05 ml min-1), percentage of acetic acid added in the mobile phase (0.05 ± 0.005%) and temperature column (65 ± 0.5°C). The calculated results showed the robustness of the procedure. The results of LP are given in Supplementary (Table S2). Similar results were obtained for SP (data not shown) and slight changes in the experimental parameters mentioned had no significant effect, confirming the robustness of the method.
Application of the method
The validated method was applied to determine the AA, Lam and Man-ol content in twelve LP and five SP kindly donated by different fertilizer companies and MAPA. All the samples were examined in triplicate (Table 3). Gives the main details provided by the manufacturers for the selected products, as well as a comparison of the declared values of AA, Lam and Man-ol (w/v% for LP and w/w% for SP) and the concentration obtained with the present methodology. As can be seen, the present studies are in good agreement, with slight differences with the declared values for the Man-ol content because the method recommended by the EU regulations is based on chromatography. However, there were substantial differences in AA content, particularly in solid products. These differences are due to the fact that fertilizer companies use a spectrophotometric method to determine their content that is less sensitive and precise than a chromatographic method [16]. The proposed methodology was in good agreement with the EU recommendations, confirming that the proposed method could be used successfully to estimate AA and Man-ol content in commercial samples with acceptable accuracy and precision. It is not usual to find the declared Lam content because this is not required by current legislation, however the present methodology will confirm its content simultaneously and provide more information about the products. In addition, these products are commonly marketed with Amino Acids (AminoA), LignoSulfonates (LignoS) or Humic Acids (HA), which may interfere in the analysis of the targets. To evaluate its influence, the developed method was applied to the analysis of a representative product of amino acids, with a composition of 36% free amino acids (AminoA), a magnesium oxide product complexed by LignoSulfonic Acid (8%, LignoS) and a concentrated Humic Extract of 15% (HA).
| Sample number | Details | Declared value | Present study | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Species | pH | Type | AA (%) | Man-ol (%) | Lam (%) | AA (%) | Man-ol (%) | Lam (%) | |
| *1 | Ascophyllum nodosum | 4.19 | Liquid | 1.32 ± 0.2 | 1.44 ± 0.03 | NS | 1.54 ± 0.1 | 1.44 ± 0.01 | 3.51 ± 0.2 |
| 2 | Ascophyllum nodosum | 10 | Liquid | 1.5 | 0.5 | NS | 1.52 ± 0.08 | 0.51 ± 0.09 | 2.73 ± 0.4 |
| 3 | Ecklonia maxima | 4.2 | Liquid | 1 | NS | NS | 0.78 ± 0.2 | <LOD | <LOD |
| 4 | Ascophyllum nodosum | 4.5 | Liquid | 7.5 | 1.7 | NS | 7.25 ± 0.2 | 1.82 ± 0.04 | 4.73 ± 0.4 |
| 5 | Ascophyllum nodosum | 3.20-3.70 | Liquid | 2 | 0.5 | NS | 1.60 ± 0.2 | 0.52 ± 0.03 | 2.52 ± 0.2 |
| 6 | Ascophyllum nodosum | 5 | Liquid | 6 | 2 | NS | 6.34 ± 0.09 | 2.33 ± 0.08 | 4.03 ± 0.09 |
| 7 | Ascophyllum nodosum | 4.3 | Liquid | 1.7 | 1.1 | 3.5 | 2.20 ± 0.3 | 1.21 ± 0.1 | 3.33 ± 0.2 |
| 8 | Ascophyllum nodosum+ Ecklonia maxima | 9.7 | Liquid | 1.5 | 0.5 | NS | 1.24 ± 0.1 | 0.47 ± 0.07 | 2.93 ± 0.08 |
| 9 | Ecklonia maxima | 4.5 | Liquid | 0.5 | NS | NS | 0.61 ± 0.1 | <LOD | <LOD |
| 10 | Ascophyllum nodosum | 4 | Liquid | 2 | 1.2 | NS | 1.86 ± 0.07 | 1.15 ± 0.03 | 3.44 ± 0.2 |
| 11 | Ascophyllum nodosum | 4 | Liquid | 1.5 | 1.09 | NS | 1.39 ± 0.3 | 0.97 ± 0.05 | 3.72 ± 0.1 |
| 12 | Ascophyllum nodosum | 8.5 | Liquid | 1.5 | 0.5 | NS | 1.27 ± 0.2 | 0.48 ± 0.08 | 1.86 ± 0.3 |
| **13 | Ascophyllum nodosum | 10.8 | Solid | 17.3 ± 0.33 | 5.68 ± 0.091 | NS | 18.0 ± 0.07 | 5.84 ± 0.10 | <LOQ |
| 14 | Ascophyllum nodosum | 9.72 | Solid | 16 | 3 | NS | 14.5 ± 0.05 | 3.12 ± 0.08 | 1.27 ± 0.1 |
| 15 | Ascophyllum nodosum | 9 | Solid | 17 | 4.1 | NS | 17.5 ± 0.3 | 4.01 ± 0.2 | 3.07 ± 0.5 |
| 16 | Ascophyllum nodosum | 8.7 | Solid | 14 | 5 | NS | 15.0 ± 0.4 | 4.89 ± 0.1 | 3.99 ± 0.1 |
| 17 | Ascophyllum nodosum | 3.7 | Solid | NS | NS | NS | 9.00 ± 0.09 | <LOQ | <LOD |
Table 3: Physicochemical characteristics of the commercial samples selected and a comparison between the declared values of AA, Lam and Man-ol (concentration expressed as percent w/v (%) in LP and w/w (%) in SP) and values obtained with the present methodology of selected samples (n=3, %RSD≤ 5% in all cases).
Representative chromatograms obtained from a LP and SP compared with representative samples of AAs, LignoS and HA is shown in Figure 3. A representative samples of products assayed were spiked with standard solution at QC2, as a result none of the other common products marketed were found to interfere with AA, Lam or Man-ol, indicating the selectivity of the method.
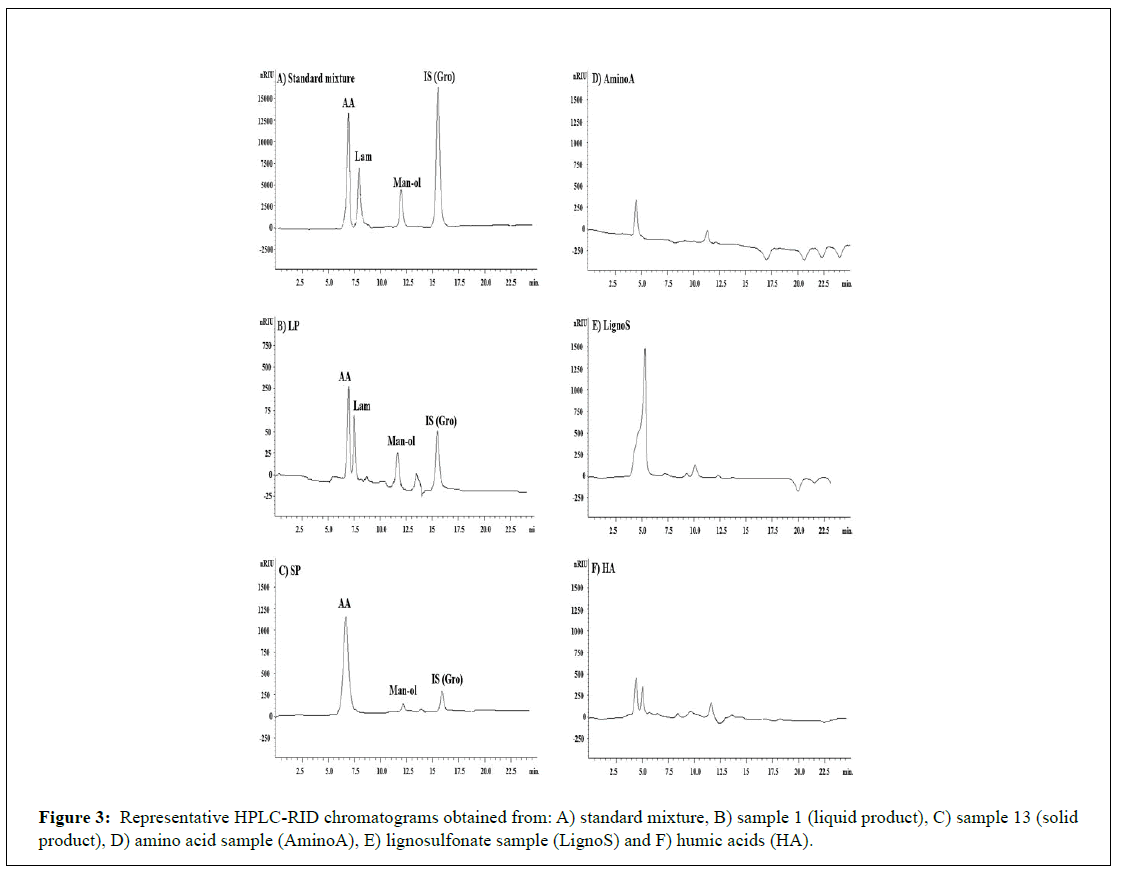
Figure 3: Representative HPLC-RID chromatograms obtained from: A) standard mixture, B) sample 1 (liquid product), C) sample 13 (solid product), D) amino acid sample (AminoA), E) lignosulfonate sample (LignoS) and F) humic acids (HA).
A simple HPLC-RID method was developed and validated to simultaneously determine AA, Lam and Man-ol in seaweed products (liquid and solid form) used as fertilizers. The proposed sample treatment was optimized and involved three simple steps: Dilution, centrifugation and filtration. Sample treatment and chromatographic analysis were achieved in 25 minutes by means of an Bio-Rad Aminex HPX-87H column with an optimized mobile phase in isocratic elution mode. The proposed method proved to be efficient and did not require the consumption of organic solvents, in line with the principles of Green Chemistry. Moreover, this method allowed for rapid determination–40 min for all analytes including sample treatment and HPLC-RID analysis– compared with the methodologies recommended by the EU where AA determination takes more than 24 hours. Additionally, RID is a cheap detector that is affordable for any laboratory. Utility of the method was demonstrated in an analysis of several commercial samples with different characteristics (algae species, pH, extraction form and AA and Man-ol content). Taking into account the EU’s recent inclusion of these products as fertilizer products in regulation CE 2019/1009, an analytical method approved by CEN is needed that can determine AA and Man-ol. In conclusion, the proposed method offers an innovative tool that allows the quality control of these analytes in seaweed fertilizers and will bwe able to help with the harmonization of CE marking. Moreover, this methodology also included the analysis of Lam, which will provide more information about seaweed extract products, and will not only be useful for quality control but also to investigate their effectiveness.
The authors are grateful to the fertilizer companies and Laboratorio Arbitral (Spain’s Ministry of Agriculture, Fisheries and Food) for kindly donating samples for this investigation.
The authors gratefully acknowledge the financial support of Spain’s Ministry of Economy and Competitiveness project: RTI2018- 096268-B-I00, and the Comunidad de Madrid (Spain) and Structural Funds 2014–2020 (ERDF and ESF) project AGRISOSTCM S2018/BAA-4330.
[CrossRef] [Google Scholar] [Pubmed]
[CrossRef] [Google Scholar] [Pubmed]
[CrossRef] [Google Scholar] [Pubmed]
[CrossRef] [Google Scholar] [Pubmed]
[CrossRef] [Google Scholar] [Pubmed]
[CrossRef] [Google Scholar] [Pubmed]
[CrossRef] [Google Scholar] [Pubmed]
Citation: Valverde S, Hernández-Apaolaza L, Lucena JJ (2022) A Simple Analytical Method to Determine Alginic Acid, Laminarin and Mannitol an Seaweed Extracts Fertilisers. J Chromatogr Sep Tech. 13:470.
Received: 01-Feb-2022, Manuscript No. JCGST-22-16167; Editor assigned: 04-Feb-2022, Pre QC No. JCGST-22-16167 (PQ); Reviewed: 18-Feb-2022, QC No. JCGSR-22-16167; Revised: 21-Feb-2022, Manuscript No. JCGST-22-16167(R); Published: 28-Feb-2022 , DOI: 10.35248/2329-9096-22.13.470
Copyright: © 2022 Valverde S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.