Journal of Physical Chemistry & Biophysics
Open Access
ISSN: 2161-0398
ISSN: 2161-0398
Research - (2024)Volume 14, Issue 1
The three-component reaction of enaminones, benzaldehyde and hydrazine-HCl or four-component reaction of enaminones, benzaldehyde, hydrazine-HCl and ethyl cyanoacetate in water in the presence of catalytic amount of ammonium acetate has been devised as a straightforward, sustainable approach for the synthesis of 1-H-pyrazole, N-aminopyridine and pyrazol[3,4-b]pyridine derivatives. The key benefit of this approach is simple experimental procedure associated with cost effective and environmentally friendly techniques.
Multicomponent reaction; Enaminones; Water; Pyrazoles; Pyrazolo[3,4-b]-Pyridine; 1,2-Bisarylidenehydrazine
Nitrogen-containing heterocycles were recognized to possess diverse activities in fields related to biological and medicinal activities [1-4]. Among nitrogen-containing heterocycles pyrazole derivatives, play an important role in agrochemical and pharmacological research [5,6].
The most extensively utilized method for pyrazole synthesis was made through condensation reaction of hydrazine with α,β- unsaturated carbonyl scaffolds [7-10].
Other commonly utilized methods for pyrazole synthesis relied on 1,3-dipolar cycloaddition of toxic explosive diazo compounds onto triple bond systems [11], or the nucleophilic attack of hydrazines to isoxazoles, flavones or chromones [12], or tosyl hydrazine with substituted aldehydes [13-15]. It is worth mentioning that although hydrazine hydrate is applied as a major source of nitrogen, however a disadvantage associated with this protocol is the usual formation of 4,5-dihydro pyrazoles which needs further oxidation to the final pyrazole derivative. Examples of biologically active pyrazole-based moieties are illustrated below in Figure 1.
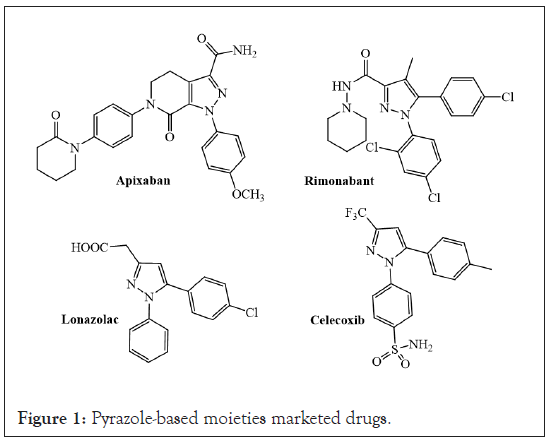
Figure 1: Pyrazole-based moieties marketed drugs.
Pyrazolo[3,4-b]pyridine derivatives acquired significant attention in medicinal chemistry for their potent and privileged range of biological activities [16-18]. Several approaches have been reported for their synthesis. The reaction of 5-aminopyrazoles with 1,3-biselectrophiles or unsaturated carbonyl compounds are among the widely used protocols [19-25]. However, these approaches usually afford dihydropyrazolo[3,4-b]pyridine derivatives. The synthesis of pyrazolo[3,4-b]pyridines has received little attention, and few approaches addressed their direct synthesis [26-29]. Although these protocols have their specific advantages, they are limited by several factors such as low yields, multistep synthetic approaches, long reaction times, tedious work up either in conducting the reaction or isolation of products, as well as the use of hazardous catalysts and solvents.
Currently, Multi-Component Reactions (MCRs) are recognized as important and reliable strategies in the rapid synthesis of complex molecules from readily available starting materials in a single step [30,31]. Therefore MCRs-based syntheses provide sustainable utility of chemical resources by reducing waste as well as minimizing the number of steps required for organic transformations [32].
Large quantities of solvents are essential for chemical reactions, extraction, and purification processes [33]. Moreover, many organic solvents are toxic, hazardous, or environmentally harmful which pose risks to both the environment and human health [34]. In this regard the use of water as a green solvent with its low ESH (Environmental Safety and Health) impact, has led to intriguing developments of organic synthesis due to its environmental benignity, safety, and low-cost nature [35-40].
As a part of our continuing work concerning the green synthesis of biologically relevant heterocycles, we report, herein a facile synthesis of novel polyfunctionally substituted pyrazoles and pyrazolo[3,4-b]pyridine derivatives via one-pot multi-component reaction of enaminones 1, benzaldehyde 2, and hydrazine dihydrochloride 3 and ethyl cyanoacetate 5 in water in the presence of catalytic amount of ammonium acetate at reflux.
General information
Aldehydes, Ethyl cyanoacetate, N,N-Dimethylformamide dimethylacetal, N,N-dimethyl acetamide dimethyl acetal and Ketones were of commercial grade and purchased from Aldrich and Merck companies. IR spectra were measured on a PerkinElmer 317 grating IR spectrophotometer using KBr pellets. 1H NMR (500 MHz) and 13C NMR (500 MHz) spectra were recorded using JEOL-E.C.A spectrometer (δ ppm). Melting points were measured on an Electrothermal melting point apparatus and are uncorrected. Mass spectrometry was performed on a JEOL-JMSAX 500 spectrometer. The appropriate precautions in handling moisture-sensitive compounds were considered. Solvents were dried by standard techniques. Elemental analyses were carried out at the Microanalysis Laboratory, National Research Center, Giza, Egypt; their values agreed favourably with the calculated ones.
Synthesis of compounds
General procedure for compounds 1a-c and 1d-f: Compounds 1a-c were prepared as described previously [41]. Compounds 1d-f were prepared via adding dropwise N,N-dimethyl acetamide dimethyl acetal (1.33 g, 10 mmol) to a stirred solution of each of acetophenone, 2-acetyl thiophene, 2-acetyl furan (10 mmol) in CH2Cl2 (10 ml). The reaction mixture was stirred at ambient temperature (25°C) for 24 hours. The solid product formed after removing solvent under reduced pressure was filtered and crystallized from cyclohexane.
3-Hydroxy-1-phenylbut-2-en-1-one 1d: Yellowish crystals, yield 1.21 g (75%), mp 86°C-88°C. IR (cm-1): 1594 cm-1 (CO); 1H NMR: (500 MHz, DMSO-d6) δ 16.30 (1H, br s, OH); 7.91-7.93 (2H, m, Ph-H); 7.49-7.93 (3H, m, Ph-H); 6.53 (1H, s, ethylene-H); 2.20 (3H, s, CH3). EIMS (m/z): 162 (M+) for C10H10O2 (162).
3-(Dimethylamino)-1-(thiophen-2-yl)but-2-en-1-one 1e: Brown crystals, yield 1.46 g (75%); mp 101°C-103°C; IR (cm-1): 1530 cm-1 (CO); 1H NMR: (500 MHz , DMSO-d6) δ 7.60-7.62 (2H, m, thienyl H-3,5); 7.06 (1H, t, J=5.0 Hz, thienyl H-4); 5.61 (1H, s, ethylene-H); 3.01 (6H, s, NCH3); 2.51 ( 3H, s, CH3). EIMS: (m/z): 195 (M+) for C10H13NOS (195).
3-(Dimethylamino)-1-(furan-2-yl)but-2-en-1-one 1f: Brown crystals, yield 1.30 g (73%); mp 94°C-96°C; IR (cm-1): 1538 cm-1 (CO); 1H NMR (500 MHz, DMSO-d6) δ 7.70 (1H, d, J=5.0 Hz, furyl H-5); 6.94 (d, 1H, d, J=5.0 Hz, furyl H-3); 6.51 (1H, t, J=5.0 Hz, furyl H-4); 5.56 (1H, s, ethylene-H); 2.99 (6H, s, NCH3); 2.52 (3H, s, CH3). EIMS: (m/z): 180 (M+1). C10H13NO2 (179).
General procedure for the preparation of 4a-c and 4d-f: To a stirred suspension of enaminones 1a-f (10 mmol) and hydrazine dihydrochloride 2 in water (10 ml), benzaldehyde 3 and ammonium acetate was refluxed under heating for 1 hr. The solid product formed after evaporation of solvent in vacuum, and trituration with ethanol was collected and crystalized from the proper solvent. In some cases, flash chromatography on silica gel using chloroform/n-hexane (3:1) as eluent was performed to afford analytically pure samples.
Phenyl(3-phenyl-1H-pyrazol-4-yl)methanone 4a: Yellow crystals, yield 1.86 g (75%); mp 160°C-162°C. IR (cm-1): 3197 (NH) and 1658 cm-1 (CO); 1H-NMR (500 MHz, DMSO-d6) δ 12.78 (1H br s, NH); 8.42 (1H, s, H-5); 8.10-8.01 (2H, m, phenyl-H); 7.91-7.51 (8H, m, phenyl-H). 13C-NMR (500 MHz, DMSO-d6) δ 188.40, 149.89, 147.14, 144.51, 139.45, 133.98, 133.86, 128.84, 128.58, 127.32, 126.94, 111.14. EIMS: (m/z): 249 (M+1) for C16H12N2O (248).
(3-phenyl-1H-pyrazol-4-yl)(thiophen-2-yl)methanone 4b: Brown crystals, yield 1.95 g (77%); mp 156°C-158°C; IR (cm-1): 3432 br (NH) and 1654 cm-1 (CO); 1H-NMR: (500 MHz, DMSO-d6) δ 7-79-7.41 (7H, m, phenyl and thienyl-H); 8.09 (1H, J=5 Hz, thienyl H-5); 8.36 (1H, s, H-5); 11.07 (1H, br s, NH). 13C-NMR (500 MHz, DMSO-d6) δ: 182.64, 149.79, 147.61, 146.53, 144.61, 134.53, 133.08, 132.56, 130.46, 129.35, 128.84, 127.44. EIMS: (m/z): 255 (M+1) for C14H10N2OS (254).
Furan-2-yl(3-phenyl-1H-pyrazol-4-yl)methanone 4c: Yellow crystals, yield 1.74 g (73%); mp 170°C-172°C; IR (cm-1): 3428 br (NH) and 1650 cm-1 (CO); 1H NMR (500 MHz, DMSO-d6) δ 7.99-7.62 (7H, m, phenyl and furyl-H); 8.09 (1H, d, J=5Hz, furyl H-5); 8.37 (1H, s, H-5); 12.47 (1H, br s, NH); 13C-NMR (500 MHz, DMSO-d6) δ: 176.99, 154.63, 150.00, 147.39, 146.69, 146.24, 134.51, 130.49, 129.38, 127.45, 115.16, 114.89, 112.71. EIMS m/z: 240 (M+2) for C14H10N2O2 (238).
5-methyl-3-phenyl-1H-pyrazole 4d: Yellow crystals, yield 0.95 g (60%); mp 124°C-126°C. IR (cm-1): 3179 (NH); 1H NMR (500 MHz, DMSO-d6) δ 12.58 (1H, br s, NH, D2O exchangeable). 7.70- 7.72 (2H, m, Ph-H); 7.23-7.34 (3H, m, Ph-H); 6.39 (1H, s, H-4); 2.21 (3H, s, CH3). 13C-NMR (500 MHz, DMSO-d6) δ: 129.13, 127.75, 125.49, 101.73, 100.0, 11.45. EIMS (m/z) 158 (M+) for C10H10N2 (158).
5-methyl-3-(thiophen-2-yl)-1H-pyrazole 4e: Brown crystals, yield 1.08 g (66%); mp 173°C-175°C; IR (cm-1): 3120 br (NH); 1H NMR (500 MHz, DMSO-d6) δ 12.50 (1H, br s NH); 7.36 (1H, d, J=5.0 Hz, thienyl H-5); 7.27 (1H, t, J=5.0 Hz, thienyl H-3); 7.02 (1H, t, J=5 Hz, thienyl H-4); 6.28 (1H, s, pyrazolyl-H); 2.20 (3H, s, CH3); 13C-NMR (500 MHz, DMSO-d6) δ: 139.74, 127.50, 124.24, 123.20, 120.4, 100.94, 54.55, 10.69. EIMS m/z: 164 (M+) for C8H8N2S: (164).
3-(Furan-2-yl)- 5-methyl-1H-pyrazole 4f: Brown crystals, yield 0.93 g (63 %); mp 162°C-164°C; IR (cm-1): 3206 br (NH); 1H NMR (500 MHz, DMSO-d6) δ 12.46 (1H, br s, NH); 7.26-7.34 (2H, m, furyl 3,5-H); 7.01 (1H, t, J=5.0 Hz, futyl H-4); 6.27 (1H, s, pyrazolyl-H); 2.21 (3H, s, CH3). 13C-NMR (500 MHz, DMSO-d6) δ: 11.01, 101.53, 123.73, 124.77, 128.03, 138.05, 140.03, 146.59. EIMS m/z: 148 (M+) for C8H8N2O: (148).
General procedure for the synthesis of 6a-b and 7: A stirred suspension of hydrazine dihydrochloride 3 in water (20 ml) and ammonium acetate (1 g), was treated with each of the enaminone 1a-c (10 mmol), benzaldehyde 2 (10 mmol) and ethyl cyanoacetate 5 (10 mmol). The reaction mixture was heated at refluxed for 1 hr, allowed to cool to room temperature, and the formed precipitate was filtered of and crystallized from ethanol to afford analytically pure samples.
Ethyl 6,7-diamino-3,4-diphenyl-4,7-dihydro-1H-pyrazolo[3,4-b] pyridine-5-carboxylate 6a: Yellow crystals, yield 1.68 g (45%); mp 160°C-162°C; IR (cm-1): 3779, 3467 and 3334 (NH2) and (NH); and 1662 cm-1 (CO); 1H NMR (500 MHz, DMSO-d6) δ 8.37 (1H, s, NH). 7.67 (br s, 2H, NH2); 7.55-7.63 (m, 5H, phenyl-H); 7.23-7.45 (5H, m, phenyl-H); 7.20 (2H, br s, NH2); 5.10 (1H, s, H-4); 4.01 (2H, q, J=7.2 Hz, CH2); 1.16 (3H, t, J=7.2 Hz, CH3); 13C NMR: (500 MHZ, DMSO-d6) δ: 193.86 (CO), 169.13 (C-6), 152.29, 148.10, 146.75, 132.06, 129.33, 129.03, 128.59, 128.80, 127.70, 126.50, 120.51, 79.06, 59.13 (CH2), 39.78 (C-4), 14.98 (CH3). EIMS m/z: 374 (M+-1) for C21H21N5O2: (375)
Ethyl 6,7-diamino-4-phenyl-3-(thiophen-2-yl)-4,7-dihydro-1Hpyrazolo[ 3,4-b]pyridine-5-carboxylate 6b: Yellow crystals, yield 2.20 g (58%); mp 237°C-239°C; IR (cm-1): 3467, 3402 and 2973 and (NH2 and NH) and 1662 cm-1 (CO); 1H NMR (500 MHz- DMSO-d6) δ 8.54 (1H, br s, NH); 7.90-8.04 (5H, m, thienyl-H and NH2); 7.40 (2H, br s, NH2); 7.07-7.19 (5H, m, phenyl-H); 5.03 (1H, s, H-4); 4.00 ( 2H, q, J=7.2 Hz, CH2); 1.13 (3H, t, J=7.2 Hz, CH3). 13C NMR: (500 MHZ, DMSO-d6) δ: 184.93, 169.08, 152.33, 147.78, 146.70, 143.81, 133.98, 133.69, 133.27, 129.23, 128.80, 128,56, 127.71, 100.0, 78.93, 59.10, 14.97 .EIMS m/z: 380 (M+-1) C19H19N5O2S: (381).
1,2-Dibenzylidenehydrazine 7: Light brown crystals, yield 0.832 g (40%); mp 93°C-95°C (lit. mp 92°C-93°C) [42]; IR (cm-1): 1669 cm-1 (C=N); 1H NMR (500 MHz, DMSO-d6) δ 7.47-7.48 (6H, m, phenyl-H), 7.85-7.86 (4H, m, phenyl-H); 8.68 (s, 2H, Benzylidinimine-H). 13C NMR (500 MHZ, DMSO-d6) δ 162.01 (C=N); 134.35; 131.89; 129.45; 128.9 EIMS m/z: 208 (M+) for C14H12N2.
General procedure for the preparation of 9a: To a mixture of hydrazine dihydrochloride 3 and ammonium acetate (1 g), suspension in water (20 ml) was added the enaminone 1d-f (10 mmol), ethyl cyanoacetate 5 (10 mmol) and benzaldehyde derivatives 8a-b (10 mmol). The resulting mixture was refluxed for 1 hr and left to cool to room temperature. The precipitated product was collected by filtration and recrystallized from ethanol affording desired products 9a, b.
1,2-bis(4-methoxybenzylidene)hydrazine 9a: Yellow crystals, yield 1.79 g (67%); mp 174°C-176°C. IR (cm-1): 1656 cm-1 (C=N); 1H NMR (500 MHz, DMSO-d6) δ 8.58 (2H, s, aldimine-H); 7.78 (4H, d, J=7.5 Hz, phenyl H); 7.02 (4H, d, J=7.5 Hz, phenyl H); 3.78 (6H, s, OCH3). 13C NMR (500 MHZ, DMSO-d6) δ 162.22 (CO-Me); 160.98 (C=N); 130.53; 127.08; 114.94; 55.92. EIMS m/z: 267 (M+-1) for C16H16N2O2: (268).
1,2-bis(3-phenylallylidene)hydrazine 9b: Light brown crystals, yield 1.82 g (70%); mp 173°C-175°C. IR (cm-1): 1643 cm-1 (C=N); MS m/z (M+-1)=259; 1H NMR (500 MHz, DMSO-d6) δ 8.35 (2H, d, J=9.50 Hz, aldimine-H); 7.62 (2H, d, J=7.15 Hz, ethylene-H); 7.39 (2H, t, J=7.15 Hz, ethylene-H); 7.25-7.35 (4H, m, phenyl-H); 7.09-7.14 (6H, m, phenyl-H). EIMS m/z: 259 (M+-1) for C18H16N2: (260).
For optimizing the reaction conditions, the three-component reaction of equimolar amounts of enaminones 1a with benzaldehyde 2 and hydrazine dihydrochloride 3 in water/ ammonium acetate was selected as the model reaction for initial studies (Table 1). We are delighted to found that pyrazole 4a was obtained in good yield when the reaction mixture was heated under reflux for 1 hour. Several green solvents including glycerol and PEG [43-48] were examined and it was found that water provided the best yield.
| Product | R | Ar | Yield % | m.p [°C] |
|---|---|---|---|---|
| 4a | H | Phenyl | 75 | 160-162 |
| 4b | H | 2-Thienyl | 77 | 156-158 |
| 4c | H | 2-Furyl | 73 | 170-172 |
| 4d | Methyl | Phenyl | 60 | 124-126 |
| 4e | Methyl | 2-Thienyl | 66 | 173-175 |
| 4f | Methyl | 2-Furyl | 63 | 162-164 |
Table 1: Description of synthesized pyrazole derivatives 4a-f.
The structure assigned for 4a was established based on analytical and spectral data. The mass the structure assigned for 4a was established based on analytical and spectral data. The mass spectrum of 4a showed a molecular ion peak m/z=249 (M++1) (55%). The 1H NMR revealed signals at δ ppm 12.78 (1H, br s, D2O exchangeable, NH), 8.42 (1H, s, H-5), 8.10-8.01 (2H, m, phenyl-H), 7.91-7.51 (8H, m, phenyl-H).
Subsequently, the scope of such reaction with a variety of enaminones has been investigated. Thus, the reaction of 1b, c with 2 and 3 performing the same reaction conditions afforded the corresponding pyrazole derivatives 4b,c.
However, the reaction of 1d, with 2 and 3 afforded product 4d with molecular ion peak m/z=158.06 (100%). Its 1H NMR revealed absorption bands at δ=12.58 (1H br s, NH), 7.70-7.72 (2H, m, phenyl-H), 7.34-7.23 (3H, m, phenyl-H), 6.39 (1H, s, H-4), 2.21 (3H, s, CH3). Structure 4d was assigned for the reaction product which confirmed the non-involvement of benzaldehyde 2 in the reaction course presented in Table 1. Similarly, enaminones 4e,f reacted with 2 and 3 yielding the corresponding pyrazoles 4e,f in moderate yields (Figure 2).
Figure 2: Multicomponent reaction of enaminones 1a-f with benzaldehyde-2-hydrazine-dihydrochloride and ammonium acetate in water.
It is worth mentioning that-and to the best of our knowledge, it is the first reported synthesis of aryl/heteroaryl (3-phenyl-1Hpyrazol- 4-yl)methanone 4a-c, while 3-aryl/heteroaryl-5-methyl-1Hpyrazoles 4d-e were synthesized via Heck reactions in a lengthy and expensive procedure [49].
To explore the generality of our procedure, we replicated our previous protocol with a four-component reaction of enaminones 1a-c, benzaldehyde 2, hydrazine dihydrochloride 3 and ethyl cyanoacetate 5. Thus, equimolar amounts of 1a, 2, 3 and 5 were heated under reflux in water/ammonium acetate, a product of molecular formula C21H21N5O2 was obtained which was assigned the corresponding pyrazolo[3,4-b]pyridine 6a based on its analytical data, which showed a molecular ion peak m/z=374 (M+- 1) (100%). The 1H NMR spectra revealed signals at δ 8.37 (1H, s, NH), 7.67 (2H, br s, NH2), 7.63-7.55 (5H, m, phenyl-H), 7.45-7.23 (5H, m, phenyl-H), 7.20 (2H, br s, NH2), 5.10 (1H, s, H-4), 4.01 (2H, q, J=7.2 Hz, CH2), 1.16 ( 3H, t, J=7.2 Hz, CH3). The 13C NMR showed characteristic bands at δ=193.86 (CO), 169.13 (C- 6), 59.13 (CH2), 39.78 (C-4), 14.98 (CH3). Similarly, the reaction mixture of 1b, 2, 3 and 5 afforded the corresponding pyrazolo[3,4-b]pyridine derivatives 6b (Figure 3).
Figure 3: Multicomponent reaction of enaminones 1a-c with benzaldehyde-2-hydrazine hydrochloride, ethyl cyanoacetate and ammonium acetate in water.
On the other hand, the reaction mixture of 1c, 2, 3 and 5 afforded the corresponding 1,2-di-benzylidine)-hydrazine 7 as a result of the reaction of two molecules of benzaldehyde with one molecule of hydrazine.2HCl (Figure 3).
Similar to the behavior of 1c toward 2, 3 and 5, the reaction mixture of 1d-f with 3, 5 and aryl aldehyde derivatives 8a, b, afforded the corresponding (1,2-di-arylidine)-hydrazines 9a, b (Figure 4).
Figure 4: Synthesis of (1,2-di-arylidine)-hydrazines 9a-b.
A reasonable mechanism to rationalize the formation of the reaction products 4a-c was depicted in (Figures 5 and 6). where 1a-c reacted with hydrazine dihydrochloride in water/ammonium acetate to yield the addition adduct 10 followed by attack of the amino group to the activated carbonyl group of benzaldehyde, afforded 11 which cyclizes to the corresponding dihydropyrazole 12, this is followed by aromatization via loss of dimethylamine in 13 to yield the final isolable products 4a-c.
Figure 5: Reasonable mechanism to rationalize for the synthesis of pyrazole derivatives 4a-c.
Concerning for enaminones 1d-f, the formed sterically hindered 1:1 adduct 14 loses the dimethylamine function, affording 15, which will cyclize to pyrazoles 4d-f via water molecule loss (Figure 6).
Figure 6: Proposed mechanism for the synthesis of pyrazole derivatives 4d-f.
Regarding the formation of 6a,b it is assumed that the condensation step of benzaldehyde and ethyl cyanoacetate afforded the in situ-formed benzylidene ethyl cyanoacetate 16, which reacted with enaminones 4a,b and hydrazine dihydrochloride yielding N-aminopyridine derivative 17. The reaction of 17 with a second molecule of hydrazine dihydrochloride yielded the final isolable products 6a-b (Figure 7).
Figure 7: Proposed mechanism for the synthesis of pyrazolo[3,4-b] pyridine derivatives 6a-b.
In summary a straightforward access to novel polyfunctionally substituted pyrazole and pyrazolo[3,4-b]pyridines were developed via three-component reaction of enaminones, benzaldehyde and hydrazine dihydrochloride or four-component reaction of enaminones, benzaldehyde, hydrazine dihydrochloride and ethyl cyanoacetate. The procedure reported herein is a simple, green and an efficient protocol. It is highly applicable and has the advantages of short reaction times and ease of execution either in conducting the reaction or isolation of products.
This work was supported by the Public Authority for Applied Education and Training of Kuwait.
Mervat Mohammed Abdelkhalik: Conceived and designed the experiments; Analyzed the Data, Wrote original and final draft. Abdulaziz Alnajjar: Writing-review and editing. Solwan Maher Ibrahim: Performed the experiments, Analyzed the Data. Mohammed Abdelmonem Raslan: Review and editing. Kamal Usef Sadek: Visualization, Methodology.
This research was done by the financial support of Public Authority for Applied Education and Training (Transform Grant TS-15-02) of Kuwait.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Abdelkhalik MM, Alnajjar A, Ibrahim SM, Raslan MA, Sadek KU (2024) A Simple and Efficient Multicomponent Synthesis of Novel Pyrazole, N-Aminopyridine and Pyrazolo[3,4-b]Pyridine Derivatives in Water. J Phys Chem Biophys. 14:371.
Received: 02-Jan-2024, Manuscript No. JPCB-24-28906; Editor assigned: 04-Jan-2024, Pre QC No. JPCB-24-28906 (PQ); Reviewed: 18-Jan-2024, QC No. JPCB-24-28906; Revised: 25-Jan-2024, Manuscript No. JPCB-24-28906 (R); Published: 01-Feb-2024 , DOI: 10.35248/2161-0398.24.14.371
Copyright: © 2024 Abdelkhalik MM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.