Journal of Clinical & Experimental Dermatology Research
Open Access
ISSN: 2155-9554
ISSN: 2155-9554
Research Article - (2022)Volume 13, Issue 4
Background: TransFORM Body Treatment with TriHex Technology® (TFB) is a topical body treatment that works in parallel with body fat reduction procedures and energy-based body skin tightening procedures to optimize results. This study aims to evaluate the efficacy of TFB when used post Radiofrequency (RF) microneedling to reduce knee skin laxity and crepey skin texture associated with aging.
Objectives: Assess the efficacy of TFB compared to a bland moisturizer used post RF microneedling treatment to the suprapatellar region in relation to improvement of skin laxity, wrinkling and elastin stimulation.
Methods: In this randomized, double-blind clinical study conducted over 16 weeks, eligible subjects underwent one RF microneedling procedure to the right and left suprapatellar region, with follow-up visits at 4, 8, 12 and 16 weeks post-treatment. Post procedure, the blinded products, were applied twice daily to the designated side, for the duration of the study. Assessments included blinded investigator and subject assessments, biopsies, standardized photography and roughness index measurements.
Results: 15 female subjects with a mean age of 52.4 years completed the study. Clinical grading measurement scores demonstrated improvement for all evaluated parameters when compared to baseline, with TFB showing statistically significant improvement in skin smoothness when compared to the bland moisturizer. Skin roughness index mirrored the improvement seen clinically and biopsies showed increased elastin generation in 5/5 slides on TFB side and 1/5 on the comparator side.
Conclusions: The use of TFB demonstrates a synergistic effect with RF microneedling related to skin smoothness and elastogenesis when used post RF microneedling of the suprapatellar. TFB appears to enhance healing and aesthetic outcomes and with ongoing use is likely to provide long term benefits.
Skin thinning and increased laxity are both undesirable consequences of aging, and the result of oxidative stresses and DNA damage that skin incurs over one’s lifetime [1,2]. One of those areas of skin thinning and laxity is the knees, where discontent is high amongst women, as such, a variety of aesthetic procedures have been developed to counteract and address the negative and undesired consequences of aging and photodamaged skin, one being microneedling. Microneedling is a common aesthetic rejuvenation procedure in which needles are inserted into the skin in a controlled manner, creating microtrauma that induces the wound healing response and with minimal damage to the epidermal layer [3,4]. In turn, collagen and elastin regeneration can be achieved, resulting in a tighter and improved appearance of aged and photodamaged skin [3-5]. Radiofrequency devices have been found to be very effective for non-ablative skin tightening, heating the dermal layer to 50-52ºC, triggering a physiologic cellular healing cascade to stimulate the formation of new collagen and elastin fibers [6,7]. These changes have been shown to improve skin wrinkles and laxity, allowing for noticeable skin tightening and lifting effects [7,8]. Combining RF with microneedling allows for effective penetration with minimal skin damage. Further, non-insulated technology enables heating of both the papillary and reticular dermis and the associated thermal denaturation, encouraging the skin to produce new collagen, while the high impedance of the epidermis allows for it to remain intact [6-8]. Current marketed RF technologies can deliver enhanced wrinkle reduction through 3-dimensional energy delivery for tissue volumizing. RF energy delivered via insulated gold-coated microneedles create precise and controllable fractionated coagulation zones within a specific layer of the dermis, and adjustable depth control allows for customized and reproducible treatments of delicate areas [6-8].
TransFORM Body Treatment with TriHex Technology® (TFB) is a marketed topical body treatment that works in tandem with body fat reduction procedures and energy-based body skin tightening procedures to optimize and compliment results [9]. TFB incorporates a combination of synergistic peptides and other actives aimed at speeding the elimination of fat particles created during the procedure, via autophagic pathways, increasing skin tone via comprehensive collagen and elastin stimulation and enhancing overall skin hydration and barrier function [9,10]. Additionally, TFB has been shown clinically as a stand-alone topical to address lax and crepey skin texture associated with aging [9-11].
The use of TFB to both optimize cosmetic procedure outcomes and address the consequences of aging skin has been demonstrated in clinical studies in which histological changes provided evidence of renewed collagen and elastin with the use of TFB [9]. Further, in a randomized comparator study conducted by Carruthers et al. TFB demonstrated greater efficacy in the production of new collagen and elastin when compared to a bland moisturizer for both the treatment and prevention of aging skin [11].
As such, this study was designed to evaluate the efficacy of TFB compared to a bland moisturizer when used post RF microneedling to the upper knees.
This randomized, double-blind clinical study conducted over 16 weeks was approved by the Institutional Review Board (IRB), IntegReview (Austin, TX). Eligible participants were healthy men and women ages 25-70, without clinically significant unstable medical conditions, that that were willing to only use the provided topical study products and refrain from extended periods of sun exposure and tanning beds, use of topical treatments, or procedures to the treatment area for the duration of the study. Exclusions to study participation included; a previous hypersensitivity or known allergy to any of the ingredients in the study products including lidocaine or other topical anesthetics, any dermatological condition or poor health that may interfere or inhibit wound healing, a previous history of hypertrophic scars, recent excessive exposure to sunlight or artificial UV light, or use of self-tanner within 7 days of study entry. Additionally, pregnancy, breastfeeding as well as participants planning on becoming pregnant during the study duration, were excluded.
Eligible, consented and enrolled participants underwent one RF microneedling treatment above the knee and were dispensed two blinded topical products labeled right and left to be applied twice daily, to the corresponding upper leg for the duration of the study. Participants returned for follow-up visits at 4, 8, 12 and 16 weeks post-procedure for assessments and photography.
Radiofrequency microneedling
At baseline, participants underwent one Lutronic® Genius RF Microneedling treatment to the upper legs right above the knee, which was administered according to the instructions for use and as determined by the Treating Investigator. Procedure protocol: NEW M Handpiece with 20 × 20 M49D tips, 2 passes, 2.5 mm and 2 mm at 10 w/300 ms.
Investigator assessments
The blinded investigator completed a global assessment of the treatment area on each leg, right above the knee, pre procedure and at 4, 8, 12 and 16 weeks post-treatment. Smoothness (wrinkles and laxity) was the parameter assessed for aesthetic outcome, while the other parameters related mostly to device effects (tone/evenness; redness; dryness/flakiness). A 5-point scale was used to assess; skin smoothness (visual), 0=smooth appearance, 4=severe, rough appearance; skin texture (tactile), 0=smooth, even feeling texture and 4=rough, uneven feeling skin texture; skin tone (evenness), 0=even, healthy color and 4=uneven, discolored appearance; skin redness/blotchiness, 0=clear, 4=severe redness; skin dryness/flakiness, 0=smooth, 4=rough and dry and overall appearance, 0=healthy, youthful skin appearance and 4=poor skin appearance.
Biopsies
A 3 mm punch biopsy of each leg, just above the knee, was collected from participants that elected to undergo biopsies, one week prior to the RF microneedling procedure, and at either week 12 or week 16 post procedures. A blinded independent dermatopathologist evaluated the specimen’s pre and post procedure for elastin generation related to skin tone.
Photography
At every visit, standardized photos were taken of the treatment area. Skin was cleansed prior to photography. 2 camera systems were utilized; LifeViz® 3D Micro (Quantificare, Inc. US, Cumming, GA) and Vectra 3D (Canfield Scientific, Parsippany, NJ). Skin roughness (or smoothness) index was analyzed by Quantificare using their software program to objectively correlate with clinical assessments.
Participant assessment
At the final, week 16 study visit, participants completed an assessment of the right and left knee/upper leg skin for overall improvement in the appearance of texture, tone and loose/lax skin. Participants designated either the right or left side or no differentiation.
Statistical analyses
GraphPad Prism Software (San Diego, CA) was used for the analyses. For the blinded investigator assessments and roughness measurements, descriptive statistics and differences in the outcome between the side treated with bland moisturizer and the side treated with TFB were computed. To test for a significant difference in the outcome between the two topicals, both parametric test (i.e. paired t test) and non-parametric test (i.e. signed rank test) were performed to ensure that the data analysis was robust regardless of which statistical methods were used. For the participant questionnaires, the percentage of each response category was computed, and chi-square tests were performed to detect any significant differences in the responses.
15 female participants mean age of 52.4 years (age range, 35-67 years), enrolled and completed the study. Fitzpatrick skin types of the participants were as follows; Type I n=1, Type II n=11 and Type III n=3.
Blinded investigator assessments
The blinded investigator graded each leg using a 5- point scale for 6 parameters. At all follow-up visits, the side using TFB had a statistically significant improvement over the bland moisturizer in visual skin smoothness (Figure 1).
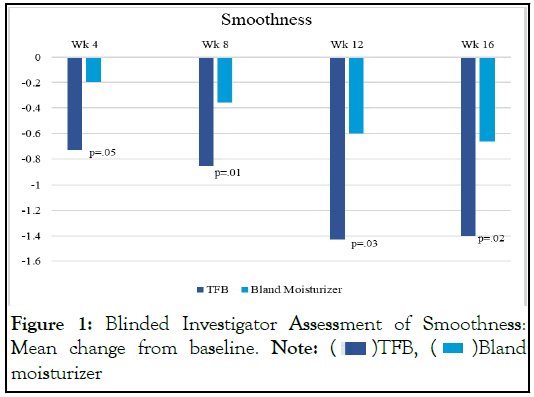
Figure 1: Blinded Investigator Assessment of Smoothness:
Mean change from baseline. Note: ![]() TFB,
TFB, ![]() Bland
moisturizer
Bland
moisturizer
Grading for skin texture, dryness/flakiness, evenness and redness/blotchiness, were generally equivalent in both groups, with TFB showing overall higher mean scores at all time points, but these were not statistically significant (Supplementary Figures). The overall appearance of the treatment area, gradually improved in the participants side using TFB, with statistical significance over the bland moisturizer at week 12, TFB -1.2 and bland moisturizer -.71 (p=0.02) (Figure 2).
Figure 2: Blinded Investigator Assessment of the Overall
Appearance of the Treatment Area: Mean change from
baseline. Note: ![]() TFB,
TFB, ![]() Bland
moisturizer
Bland
moisturizer
Objective photographic analysis
Using 3D photographic software, skin roughness and irregularity was calculated on each side and compared to baseline. As shown in Figure 3, the TFB treated side continued to have a decrease in roughness with statistical significance over the bland moisturizer side at week 16. TFB -.326 and bland moisturizer .013, p=.02 (Figure 3).
Figure 3: Objective Roughness Measurement of the Treatment
Area: Mean change from baseline.![]() TFB,
TFB, ![]() Bland
moisturizer
Bland
moisturizer
The clinical appearance confirmed roughness index measurements with skin looking smoother on the TFB side at week 16.
Participant assessment
At the final visit, participants assessed which side looked more improved and appeared tighter. 64% of participants chose the TFB side for more improvement and “skin looks tighter”. 36% participants chose the bland moisturizer side for more improvement and “skin looks tighter” (Figure 4 and 5).
Figure 4: Female age 35: right side TFB, left side bland moisturizer (a) Baseline (b) Week 16.
Figure 5: Female age 51: right side bland moisturizer, left side TFB (a) Baseline (b) Week 16.
Biopsies
Elastin staining using Movat stains was carried out in 5 patients. In particular the dermatopathologist concentrated on differences in elastin generation. The most significant changes on the TFB side, was the transformation from small fragmented elastin fibers to fully form healthy fibers and a general increase in elastin. This occurred in 5 out 5 cases on the TFB side as opposed to 1 out 5 on the comparator side (histological examples Figures 6 and 7 and all biopsies displayed in supplementary information).
Figure 6: Movat stain for elastin (a) Bland moisturizer: left baseline, right week 12 (b) TFB; left baseline, right week 12.
Figure 7: Movat stain for elastin (a) Bland moisturizer: left baseline, right week 12 (b) TFB; left baseline, right week 12.
Laxity above the knees is a common complaint among women. The anti-gravitational anatomic position paired with a natural aging phenomenon aggravated by chronic sun exposure in some patients, make this an area particularly prone to loose, crepey skin. RF microneedling is a modality designed to tighten skin through denaturation of components within the Extracellular Matrix (ECM) with stimulation of new collagen, and to a certain extent elastin. The combination of RF microneedling with the TFB product was tested in particular, for the capacity of TriHex Technology® to stimulate new elastin, a vital component to skin smoothness and elasticity [11]. In addition, products with TriHex Technology® have demonstrated improved healing effects on skin treated with various ablative and non-ablative procedures [12].
In this study, the healing process and side effect profile related to RF microneedling treatment is minimal, and thus parameters relating to healing were not of major consequence. Thus, investigator assessment relating to redness, evenness, and dryness were not significantly different between the two sides. However, the smoothness assessments, particularly in the later study visits, were important as this relates to the skin laxity and wrinkles that are the main reason for seeking treatment. The RF microneedling treatment itself is expected to have the primary positive effect on these parameters but should the topical treatment stimulate further elastin formation and ECM remodeling, this would be well represented by investigator assessment of smoothness, quantified analysis of the ‘roughness index’ and biopsy evidence of changes in the ECM showing increased elastin levels. This was the outcome demonstrated in this study. The biopsy evidence of elastin change was particularly noteworthy - one would anticipate a certain amount of neoelastogenesis from the device itself but there is a stark difference in elastin generation, evident in these biopsy sections that can largely be attributed to the TFB topical in analysis of the differences observed between the 2 sides.
An important limitation to mention here is the dependence on these types of studies on completely subjective assessments by patients in very difficult circumstances where the device is expected to play a major role. Demonstrating a difference in outcomes is thus a challenge in these situations and the addition of objective analytic tools is essential to the process. Thus, the roughness index measurement (far more accurate than the naked eye) and the biopsy results, provide a necessary validation step to the process. Additionally, a topical would be expected to be the adjunct to the device creating a long-term synergy for ongoing improved ultimate results. In this study, the stimulation of elastin in all biopsy specimens examined, almost exclusive to the TFB side, bodes well for ongoing skin tightening effects. The device provides the periodic (probably repetitive) powerful boost to the skin elasticity while the topical provides longer term incremental beneficial effects.
RF microneedling was performed for the treatment of laxity and wrinkling to the suprapatellar region on both legs. Post procedure, an adjunctive topical therapy, with actives aimed at elastin stimulation and skin tightening, (TFB), was a pplied to one side, and the other side applied a bland moisturizer. TFB appeared to add a component of skin tightening to the procedure that manifests as statistically significant increased smoothness. Additionally, photographic measurements of skin roughness, showed an improvement on the TFB side over the control. Biopsy analyses validated an increased elastin deposition in the ECM. In summary, TFB demonstrated a synergistic effect when used post RF microneedling of the suprapatellar region.
[CrossRef] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
Citation: Robinson DM, Giannini A, Rahman SM, Bell M, Widgerow A (2022) A Single Center, Randomized, Double-Blind Study to Evaluate TransFORM Body Treatment with TriHex Technology in Combination with Radiofrequency Microneedling to the Suprapatellar Region. J Clin Exp Dermatol Res. 13:618.
Received: 09-Sep-2022, Manuscript No. JCEDR-22-19150; Editor assigned: 13-Sep-2022, Pre QC No. JCEDR-22-19150 (PQ); Reviewed: 27-Sep-2022, QC No. JCEDR-22-19150; Revised: 04-Oct-2022, Manuscript No. JCEDR-22-19150 (R); Published: 14-Oct-2022 , DOI: 10.35248/2155-9554.22.13.618
Copyright: © 2022 Robinson DM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.