Journal of Research and Development
Open Access
ISSN: 2311-3278
ISSN: 2311-3278
Research Article - (2024)Volume 12, Issue 2
The water which contains high concentration of bicarbonates chlorides, sulfates and nitrates of calcium and magnesium is hard water. Water is the essence of life but water with high degree of hardness is of no use for domestic and industrial applications. Six samples of ground water have been collected from selected area-six villages from Nagpur district, Maharashtra, India. The hardness of water samples is determined by EDTA titrimetric method. The result of all tested samples shown that all samples (A-E) were very hard in nature. The hardness level of Water Sample-D is high as compared to other water sample. The present Study did not reveal any soft water. The present study aware to public to know about the degrees of hardness in underground water and its effects.
Titrimetric method; Underground water; EDTA; Hardness
Hardness in water is due to the presence of dissolved salts of calcium and magnesium. It is unfit for drinking, bathing, washing and it also forms scales in boilers. Hence it is necessary to estimate the amount of hardness producing substances present in the water sample. The estimation of hardness is based on complexometric titration. Hardness of water is determined by titrating with a standard solution of ethylene diamine tetra acetic acid (EDTA) which is a complexing agent [1-5]. Since EDTA is insoluble in water, the disodium salt of EDTA is taken for this experiment. EDTA can form four or six coordination bonds with a metal ion. Two types of hardness are present in water, temporary hardness and permanent hardness. Temporary hardness is due to the presence of bicarbonates of calcium and magnesium ions. It can be easily removed by boiling. Permanent hardness is due to the presence of chlorides and sulphates of calcium and magnesium ions which cannot be removed by boiling. The United States National Research Council has found that hard water can actually serve as dietary supplements for calcium and magnesium (NRC, 1974). Hard drinking water is generally not harmful to one’s health but can causes serious problems in domestic and industrial settings [6-8]. There were some undesirable effects observed due to the salts present in the water [9-11]. In domestic setting; more soap is required for washing and bathing, more time and more fuel required for cooking due to hard water, In Industrial setting; affect the quality of the paper in paper industry due to calcium and magnesium salts present in the water, affects the crystallization of sugar while water consisting sulfates, nitrates and carbonates in sugar industry, spoil the desired shade of the cloths in dyeing industry and also destroys the fabrics in textile industries [12-14]. Due to 100 mass molecular weight and 50 mass equivalent weight of calcium Carbonate, the hardness constructing salts conveyed in the terms of total equivalent mass weight of CaCO3.
It’s expressed in mg/l,
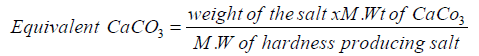
Degree of hardness is expressed in the units are Parts per million or Milligrams per liter or Clarke’s Degree French Degree.
Relationship in between the degrees of water Hardness-PPM=mg/lit=0.1o Fr=0.07o Cl.
TIn the given experimental method, total hardness was measured by the Ethylenediamine Tetraacetic Acid (EDTA) method for hard water samples collected from different areas [1-5]. All the water samples (A-F) were collected and stored in to sterilized screw capped glass bottles and brought to the laboratory. Hardness of the Water Sample-A was tested by using EDTA-tritrimetric method by taking 10 ml of water sample (without boiling) into a conical flask along with 3 ml of ammonia buffer solution and 2-3 drops of Erichrome Black-T indicator followed by titration with EDTA solution present in a burette. End point is noted down when colour of solution changes from wine red to blue and expressed as CaCO3 equivalent in mg/l or ppm which indicates that temporary hardness present in water. Similarly, the boiled and filtered water was tested by using EDTA-tritrimetric method which shows the presence of permanent hardness in water (Table 1).
| Sr. No. | Classification | Hardness in mg/L | Hardness in ppm |
|---|---|---|---|
| 1 | Soft | 0-60 | less than 60 |
| 2 | Moderately hard | 61-120 | 60-120 |
| 3 | Hard | 121-180 | 120-180 |
| 4 | Very hard | ≥ 181 | >180 |
Table 1: Types of hardness according to United States geological survey.
The remaining water samples (B-F) were tested by same procedure and results of hardness for water samples shown in Table 2.
| Sample no. |
Location (Sample water) |
Type of water | Total hardness |
|---|---|---|---|
| A | Panchgaon | Well water | 400 ppm |
| B | HudkeshwarKhurd | Bore well water | 535 ppm |
| C | Dhamana | Bore well water | 550 ppm |
| D | Chikana | Bore well water | 620 ppm |
| E | SalaiGodhani | Well water | 430 ppm |
| F | Chimnazari | Well water | 490 ppm |
Table 2: Location and hardness of different ground water samples.
During this process, the EDTA reacts with the Ca2+ and Mg2+ ions and to form the EDTA-Ca and EDTA-Mg complex (Figure 1), and after the completion of this reaction, the wine red color disappears slowly because the EBT-Ca and EBT-Mg are replaced and form stable Ca-EDTA and Ca-EDTA, colorless complex, while simultaneously blue color appears due to the effect of the EBT indicator (Figure 1).
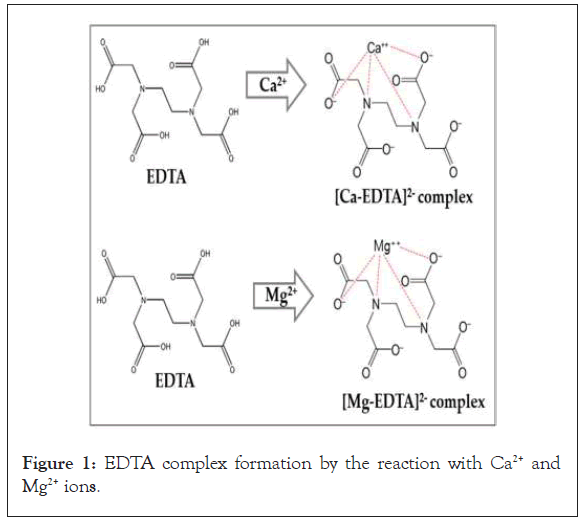
Figure 1: EDTA complex formation by the reaction with Ca2+ and Mg2+ ions.
The qualitative analysis carried out on underground water samples collected from six selected areas in Nagpur District; viz-Panchgaon village (sample-A) Hudkeshwer Khurd village (Sample-B), Dhamana village(Sample-C), Chikna villege (Sample-D), Salai Godhani villege (Sample-E) and chimnazari village (Sample-F).
The Hardness of the water samples were tested by using EDTA-tritrimetric method and the end point/results expressed as CaCO3 equivalent in mg/lor ppm (Standard methods). The results of water samples had shown the presence of Ca2+, Mg2+ ions. It is therefore in order to say that all the water samples contain both temporary and permanent hardness and the statement is confirmed and indicated by Table 1.
The present study had revealed that the water sample-A from Panchgaon village shown the hardness level 400 ppm of CaCO3 i.e., the sample is very hard in nature. In Hudkeshwar khurd village, the hardness level of water Sample-B had shown 535 ppm of CaCO3. Similar type of result was found for Sample-C of Dhamana village i.e., 550 ppm of CaCO3 i.e. water is very hard. The hardness level i.e., 620 ppm of CaCO3 shown by the water Sample-D in Chikana village.
In Salai Godhani village and chimnazari village, the water Sample-E and water Sample-F shows the high levels of hardness 430 ppm and 490 ppm of CaCO3 respectively. The results of all selected areas were different. This is due to the fact that temporary hardness of water is caused by the presence bicarbonates of carbonates of either calcium or magnesium. While permanent hardness of water is brought about by the presence of chlorides and sulphates of calcium and magnesium. Hard water does not readily form lather with soap due to the presence of the dissolved salts of Calcium and magnesium.
Boiling and addition of lime (Ca(OH)2) and washing soda (Na2CO3) were the suitable methods used in removing the hardness. This is because boiling is a very simple process while washing soda is cheap and available. During the research work, it was found that when 0.1 M of soap solution was titrated against 25 cm³ of sample-A before boiling, then lather formation started after the consumption of 20 cm³ of the soap solution which was indicating the presence of both temporary and permanent hardness in the sample. But when 0.1 M of soap solution was titrated against 25 cm³ of sample-A after the boiling and filtration of sample-A, then the appearance of lather was observed after the consumption 12 cm³ of the soap solution. This showed that temporary hardness was successfully removed by boiling and filtration process. Similar results were obtained for other sample (B-F) with slight changes as presented in Table 2.
The present study has proved the degree of hardness in collected water samples which may be harmful to the people. But the use of hard water does not give any evidence to prove casualty among those people who are using hard water. Excess of calcium present in water is not good for bones as it causes extra growth of bones especially in the back bone and become a big problem. This is important to bring awareness among the people about soft, moderately hard, hard and very hard water. There are both positive and negative effects of using extreme hard water hence awareness must be brought among the public.
The authors would like to acknowledge the warmest thanks to Dr. Salim Chavan, Principal, Govindrao Wanjari college of Engineering & Technology, Nagpur and Dr Pravin Gaidhane, Head, Department of Chemistry, GWCET, Nagpur for providing necessary laboratory facilities in college campus for research work. The authors are also thankful to laboratory supporting staff for their constant support during research work.
Citation: Ishwarkar SA, Gaidhane PK, Aayade PS, Gaidhane MK (2024) A Study of Hardness in Underground Water in Selected Areas in Nagpur District, Maharashtra, India. J Res Dev.12:257.
Received: 01-May-2024, Manuscript No. JRD-24-31026; Editor assigned: 03-May-2024, Pre QC No. JRD-24-31026 (PQ); Reviewed: 20-May-2024, QC No. JRD-24-31026; Revised: 27-May-2024, Manuscript No. JRD-24-31026 (R); Published: 03-Jun-2024 , DOI: 10.35248/2311-3278.24.12.257
Copyright: © 2024 Ishwarkar SA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.