Andrology-Open Access
Open Access
ISSN: 2167-0250
ISSN: 2167-0250
Research Article - (2021)Volume 10, Issue 8
Background: There is limited knowledge and research regarding male contraceptives. Surveys conducted by pharmaceutical companies have shown that males will not take a pill that will affect their hormones. However, data from a government survey conducted in the United Kingdom in 2019 showed that 33% of sexually active men agreed on taking a male contraceptive pill, which is the exact same percentage of women that currently use a hormonal contraception. This research project seeks to establish whether the stated evidence regarding Slo1 and Slo3 as potential targets is adequate, whether there is evidence of the expression of Slo1 and/or Slo3 cellular localization in human spermatozoa and whether it can lead to the development of a male contraceptive pill.
Methods: A systematic review on numerous databases was conducted. The searched literature was limited to the English language, Humans, Mice, Vertebrates, Systematic Reviews, Meta-analyses, clinical trials, Randomised Controlled Trials (RCTs), animal studies, cohort studies, case-control studies, and qualitative studies. The studies that were considered relevant were then assessed for their eligibility via an amended Downs and Blacks checklist.
Conclusion: Slo1 is highly expressed in the aorta, which should be kept in mind when developing a male contraceptive. Slo1 is not only found in sperm, but it is also present in twelve different tissues. This could present a huge challenge as separate isoforms mean separate proteins. If these are all in sperm, the specificity of the potential male contraceptive may be for different isoforms. The Slo3 channels are involved in various mechanisms affecting male fertility, making Slo3 a potential target for a male contraceptive pill. More studies are needed to further our understanding of Slo3 and Slo1, as there are a lot of gaps in knowledge and not enough studies focusing primarily on humans.
Reproductive; Hormonal level; Sperm motility; Progesterone
ZP: Zona Pellucida; CatSper: Cation Channel of Sperm; Vm: Membrane Potential; KSper: Potassium Current in Sperm; WT: Wild Type; mSlo3: mouse Slo3 Channel; hSlo3: human Slo3 Channel; mSlo1: mouse Slo1 Channel; hSlo1: human Slo1 Channel; AR: acrosome reaction; RCTs: Randomised Controlled Trials
History of male contraceptive pills
The development of a male contraceptive started the same time as the development of the hormonal female contraceptive methods, and they were mainly targeting hormonal levels. Historically contraceptive pills have been largely hormonal in nature as we can see with the female contraceptive pill methods. There is a difference in the physiology and reproductive biology amongst males and females. A male contraceptive should decrease the number of the fertile sperm that are ejaculated into the vagina; in a way that fertilisation is prevented. There is also an important recession regarding the financial support by the pharmaceutical industry in terms of the development of a male contraceptive. Some reasons behind the recession are the uncertainty from regulatory agencies, men’s willingness to use new contraceptive methods, their ability to deal with unwanted side effects and their female partners relaying on them for their use Thirumalai et al. [1]. Surveys conducted by pharmaceutical companies have shown that males will not take a pill that will affect their hormones, even though females have been doing it for years Heinemann et al. [2]. However, data from a government survey conducted in the United Kingdom in 2019 showed that 33% of sexually active men agreed on taking a male contraceptive pill, which is the exact same percentage of women that currently use a hormonal contraception. There was an agreement between both British males and females as 79% agreed that the appropriate use of contraception is the equal responsibility of both genders. Both genders (31% males and 33% females) have been concerned about the side effects the contraception methods have. In women, the general side effects are the main factor that deters them from using a hormonal contraception. Whereas with men, the main factor behind their reluctance is them being uncomfortable with the concept of an implant [3]. It is important to note that in the United Kingdom 54% of women rely on male contraceptive methods as their main method of contraception such as sterilisation, use of condoms and/or the use of practice coitus interruptus, which is not considered a form of contraception by all Reynolds et al.
Criteria for a good contraceptive pill
A good contraceptive chigh efficacy. The effect the contraceptive has must be reversible as this is on demand and there are already permanent methods of contraception in the market. The contraceptive should offer protection with minimal time delay. It should also be easy, simple, and accessible to all to use. The most important criterion for a good contraceptive pill is minimal side effects regarding the individual’s health, sexual pleasure, and function Reynolds et al. It should also have no impact on an eventual offspring and can be accepted easily by both partners Gava et al. The WHO has several medical eligibility criteria for contraceptive use. These criteria are all for female contraceptive methods and the only available criteria for men are regarding surgical sterilization [4-6].
Why should the ion channels be targeted?
Ion channels in spermatozoa control and regulate the intracellular ionic concentration and the membrane potential. They are proteins that allow the transport of ions across cellular membranes. Gating is the process where ion channels will undergo conformational changes that are triggered by the changes in the membrane voltage. Ion channels that are voltage gated rely on the voltage gradient within the plasma membrane to close or open. Certain ion channels will allow the passage of ions that are charged negative (anion) or positive (cation). Ion channels that are voltage gated are distinguished on the specific conductivity of the ions, for example the Ca2+, K+ and Na2+ channels. There is another type of ion channel, known as ligand gated, where the channels close or open depending on the binding of a ligand to the channel. The categorization of ligand gated ion channels is based on the principal signalling transmitter Shukla et al. (Figure 1) [7].
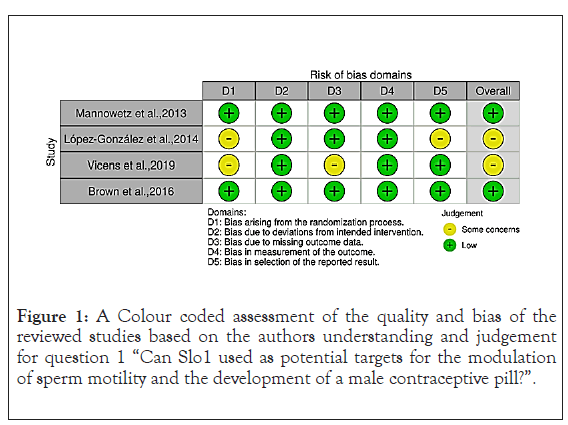
Figure 1: A Colour coded assessment of the quality and bias of the reviewed studies based on the authors understanding and judgement for question 1 “Can Slo1 used as potential targets for the modulation of sperm motility and the development of a male contraceptive pill?”.
Ca2+ channels are one of the most essential for the regulation of sperm motility in Figure 2. Ca2+ channels are a secondary messenger aiding in the activation of signalling pathways that control motility. Sperm motility, activation and its maintenance rely on the intracellular pH, the adequate balance of intracellular ions and the membrane potential. Ca2+ is also essential for the preservation of the mitochondrial functioning in sperm and the production of ATP. Equally, Ca2+ is essential for motility in the sperm and its concentrations must be conserved at sufficient levels for the maintenance of motility. This maintenance is controlled by different calcium channels Nowicka et al. In sperm there are three Ca2+ associated channels: Ca2+ ATPase, CatSper, and the Na+ and Ca2+ exchanger. The entrance of Ca2+ to the spermospore to activate sperm motility occurs due to CatSper. Ca2+ ATPase eliminates intracellular Ca2+ and allows the entry of H+ into the sperm. The Na+ and Ca2+ exchanger moves the Ca2+ ion outside of the sperm and permits the entrance of three Na+ ions. This is important for the maintenance of the balance of the intracellular environment of the Ca2+ Sun et al.
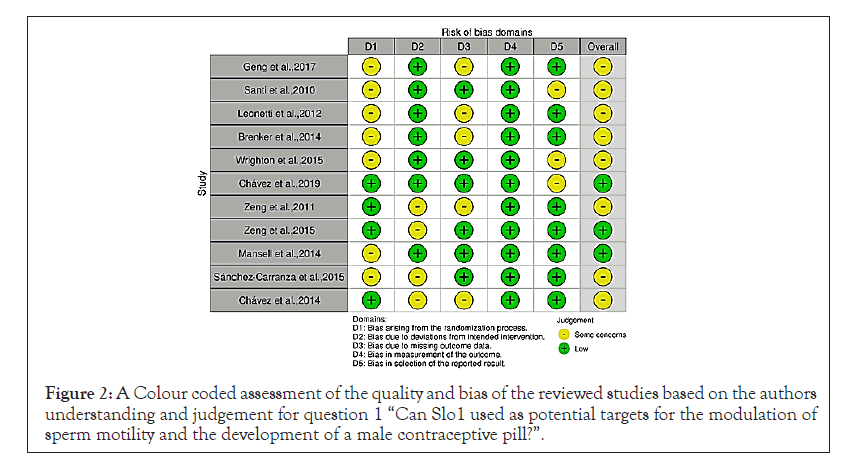
Figure 2: A Colour coded assessment of the quality and bias of the reviewed studies based on the authors understanding and judgement for question 1 “Can Slo1 used as potential targets for the modulation of sperm motility and the development of a male contraceptive pill?”.
K+ channels focusing on Slo1 and Slo3
Plasma hyperpolarisation is essential for the instigation of sperm motility and hyperactivation. Plasma hyperpolarisation occurs from potassium channels found in sperm. The most portrayed K+ channels in sperm, are Slo1 and Slo3. Slo1 and Slo3 belong to the Slo gene family and are voltage gated K+ channels. K+ channels regulate the membrane potential (Vm) and osmolality in cells. Membrane depolarization activates these channels in sperm Nowicka et al. The activity that occurs in potassium channels is thought of as regulating sperm via a Ca2+ increase Brown et al. In human sperm an increase in K+ leads to an increase in Ca2+. Also, the human K+ currents are unaffected by intracellular alkalinization and rely on intracellular Ca2+. Intracellular Ca2+ is a result of capacitation Mannowetz et al. The human K+ channels are sensitive to both Ca2+ and pH Geng et al. In mice, Slo3 is located in the principle piece and it is essential for fertility Brown et al. Slo3 in mice is activated by alkalinization and is unaffected by Ca2+ Mannowetz et al. Evidence from mice deficient in Slow (Slo3-/- mice) has demonstrated that sperm is less motile and unable to fertilise the egg in the absence of Slo3. Mice spermatozoa that lacked Slo3 were not as motile when compared to mice sperm where Slo3 was present and were unable to fertilise the egg. This could be a result of the absence of membrane hyperpolarisation, resulting in the absence of the activation of CatSper. Similar effects have not yet been observed in human sperm as there is a gap in knowledge surrounding Slo3 and the way it affects fertility Nowicka et al. It is assumed that Slo1 and Slo3 have the same effects in humans as they have in mice but that has not yet been proven. An elimination of the Slo3 gene in mice will lead to drastic changes in sperm such as: a reduction in the progressive motility, a weakened acrosome reaction, morphological abnormalities that occur after capacitation and the occurrence of membrane depolarization throughout capacitation. This implies that the Slo channels are important for male fertility in mice. This makes them a probable candidate of being a major channel in human sperm. However, as there are differences between mice and human models, there should be caution when conducting studies using mice as a model for humans. The study Mannowetz et al. conducted did provide crucial information that may aid in the making of a male contraceptive pill however, when looking at the methodology and the participants used, the data were all focused on 19 healthy fertile male volunteers that were aged 21-38 years old and to male mice. The number of individuals used is not adequate as there is a variation in reactions. The individuals were also described as healthy meaning that they had functioning sperm, thus there were not that many issues to target. When developing a male contraceptive, it should not be based solely on data from healthy individuals. Brown et al. used 107 volunteer donors 40 of them were IVF patients, 41 ICSI patients and 26 normozoospermic donors [8-11]. This data does give us a broader approach, but 107 volunteers may be not adequate number to represent all, more individuals should be tested focusing on all sperm related issues.
In humans, Slo1 is activated by Ca2+ whereas Slo3 is activated by alkaline pH. Both Slo1 and Slo3 are made up by a few auxiliary subunits and four α subunits Nowicka et al. Slo1 and Slo3 are dispersed throughout the body and respond to different stimuli Wrighton et al. [12]. Using the western blotting technique Mannowetz et al. confirmed that Slo1 appeared in the tails of human sperm. This study also stated that progesterone can block Slo1. The blockage of K+ channels lead to the complete opening of Ca2+ channels, which leads to an influx of Ca2+ ions, inducing hyperactivation. In the Slo1 there is a cytosolic C-terminus amongst two intracellular regulators of K+ conductance. These regulators enclose Ca2+ binding sites which have a high affinity. This allows the Slo1 channels to recognise the alterations in the intracellular Ca2+ and the voltage. This increases the chance of Slo1 being the channel that denotes human KSper (K+ current) Mannowetz et al. KSper is a major ion current in capacitated sperm, as it is pH sensitive and controls the sperm membrane potential Navarro et al. Three Slo1 inhibitors: charybdotoxin, paxilline and iberiotoxin inhibit the potassium levels in human sperm. Paxilline does not affect the Slo3, unlike the others Mannowetz et al. The Slo3 channels are not sensitive to Ca2+ but are susceptible to intracellular alkalinization. However, Brenker et al (2014), stated that human Slo3 is weakly activated by pH and is activated more powerfully by Ca2+. This means that the Vm is regulated more powerfully by Ca2+ than pH. This study also stated that Slo3 is the main K+ channel in human sperm and that there are important differences between mice sperm and human sperm. Whilst Mannowetz et al. indicated that Slo1 is the Ca2+ activated K+ channel in human sperm, Lopez-Gonzalez et al. stated that both Slo1 and Slo3 aid in the hyperpolarisation that occurs during capacitation. Proteomic evidence, however, indicates that Slo3 is the principal K+ channel in human sperm Brenker et al. [13-15]. Further studies will have to be conducted to examine which K+ channel is essential in human sperm.
Systematic literature search and search terms
A systematic literature search was conducted on the 5th of April 2021 on published studies up to this year, 3rd July 2021 using the PubMed, Medline, and the Cochrane database. The search was based on the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines Page et al. [16].
The studies that were grouped for synthesis were: clinical trials, Randomised Ccontrolled Trials (RCTs), animal studies, cohort studies, case-control studies, qualitative studies, meta-analysis, and systematic reviews. The systematic literature search was conducted by a single author only. Studies that were not written in English were excluded. Studies had to be conducted on human, mice and/or vertebrates. Studies before 2010 were excluded. They were also excluded if there was no full text article and were published as abstracts only. All the titles and abstracts of the studies were read and assessed and if they were considered relevant following the inclusion and exclusion criteria, to either of the research questions they were then assessed for their eligibility via an amended Downs and Blacks checklist Downs et al. [17]. The chosen studies and their references were reviewed and assessed. The main points of interest in all the chosen studies were whether slo1 and slo3 can be targeted, to what extent, how can they be targeted, their effects on sperm motility, their effect on fertility and their effects on other parts of the human body. The studies focused on fertile mice, infertile mice, human male factor infertility, fertile human males. All studies that focused only on female factor infertility were excluded.
The conducted systematic literature search can be seen on the provided PRISMA flow diagrams. All studies were assessed based on an adapted “Downs and Black standardised checklist” (see Appendix 1) Downs et al. The checklist provides points and questions aiding in the assessment of the quality, relevance, and limitations of the searched studies. The studies found from the PubMed, Medline and Cochrane databases search were colour coded and the tables can be seen below: red indicated that the study could not answer the research question and/or there was a high risk of bias, yellow that it could answer it to a certain extent or that it gave general information regarding Slo1/Slo3 and/or the risk of bias was undetermined and green indicated that it directly answered the question and/or there was a low risk of bias. Some studies provided raw data; these were also summarised based on their significant results (p<0.05).
Literature search
Can Slo1 be used as potential targets for the modulation of sperm motility and the development of a male contraceptive pill: The first database search generated 21 studies, from these studies 13 were assessed for their eligibility? Excluded studies are depicted below and the included studies have been colour coded based on their bias assessment as seen in. The included studies have been tabulated based on their author, intervention, study type, indication, reported outcomes and their results (Table 1).
| Author | Intervention | Study type | Indication | Reported outcomes | Statistical analysis |
|---|---|---|---|---|---|
| Mannowetz et al., 2013 | Electrophysiology Immunocytochemistry Electrophoresis Immunoblotting Western Blot analysis | Case-control Animal | 19 Healthy Fertile human volunteers (21-38 years old) Male C57BL/6 mice | hKSper is sensitive to Slo1 channel blockers charybdotoxcin, paxilline and iberiotoxin Slo1 is present in human spermatozoa. | No data presented. |
| López-González et al.,2014 | Flow Cytometry Subcloning Western Blot analysis Immunodetection | Case-control | Human Sperm Hyperpolarisation in human sperm depends on the K+ permeability increases which is affected by the inhibitors in Slo3 and Slo1 channels. | Slo3 channels can be found in human sperm. | p<0.05 and p ≤ 0.05 when compared to the control. |
| Vicens et al.,2019 | Sequence’s retrieval and multiple alignment Phylogenetic analysis | Case-control | Protein coding sequences | Slo3 is less conserved than Slo1 | No data presented. |
| Brown et al.,2016 | Electrophysiology | Case-control | Healthy volunteer donors and sub fertile IVF and ICSI patients (40 IVF patients, 41 ICSI patients and 26 normozoospermic donors) | Spermatozoa with abnormalities in their Slo1 electrophysiology had a normal respond to progesterone. They also had high Ca2+. | p <0.02 p =0.012 |
Table 1: A summary of the studies included for the research question “Can Slo1 be used as potential targets for the modulation of sperm motility and the development of a male contraceptive pill?” in this systematic review [9,10,14,18].
Can Slo3 be used as potential targets for the modulation of sperm motility and the development of a male contraceptive pill: The first database search generated 49 studies, from these studies 43 were assessed for their eligibility? Excluded studies are depicted below and the included studies have been colour coded based on their bias assessment as seen in. The included studies have been tabulated based on their author, intervention, study type, indication, reported outcomes and their results (Table 2).
| Author | Intervention | Study type | Indication | Reported outcomes | Statistical analysis |
|---|---|---|---|---|---|
| Brenker et al.,2014 | Mass spectrometry Immunocytochemistry Western Blot analysis Patch-clamp Recording | Case-control | Healthy volunteers | Main K+ channel in humans is controlled by Ca2+ Slo3 and LRRC52 are expressed in human sperm Intracellular Ca2+ does not activate mouse Slo3. | No data presented. |
| Wrighton et al.,2015 | Electrophysiology | Case-control | mSlo3 sequence R196Q F304Y | Ba2+ blocks Slo3 Quinine and quinidine inhibit Slo3. | p <0.0001 |
| Chávez et al.,2019 | PCR product cloning Western Blot analysis | Case-control | CD1 male mouse | Slo3 is expressed in mice isoforms. | No data presented. |
| Chávez et al.,2014 | Ca2+ imaging pH imaging | Case-control Animal | Slo3 knockout mice | Alkaline depolarization in wild-type and Slo3 mutant sperm can evoke internal increase of Ca2+ increase depends on Slo3 activity after capacitationIn Slo3 mutant sperm there is a deficiency in the increase of Ca2+. | No data presented. |
| Zeng et al.,2011 | Electrophysiology | Case-control Animal | Mice Slo3 knockout mice 6 wild type male breeders | Most alkalisation activated currents are abolished by Slo3 deletions Slo3 in spermatozoa is essential for alkalization activated hyperpolarisation Slo3-/- male mice are Osmoregulation is affected by the absence of Slo3.Epididymal sperm from Slo3-/- mice show deficits in their motility.Capacitated Slo3-/- show partial IVF success.infertile. | No data presented. |
| Zeng et al., 2015 | Electrophysiology | Case-control | LRRC52-/- mice | LRRC52-/- mice have severe impaired fertility. | p<0.005 |
| Mansell et al., 2014 | Electrophysiology | Case-control | Volunteer donors with no know fertility problems after 48-72h of sexual abstinence. | Channel activity was increased by quinidine, progesterone, and 4-AP. | p <0.001 |
| Tang et al.,2010 | Electrophysiology | Case-control | Slo1 and Slo3 | Quinidine blocks Slo3 | No data presented. |
| Geng et al.,2017 | Electrophysiology | Case-control | hSlo3-WT cDNA | Slo3 in humans has a low conservation. | p ≤0.001 |
| Leonetti et al.,2012 | Electrophysiology | Case-control | hSlo3 | hSlo3 is pH sensitive | No data presented. |
| Santi et al.,2011 | Western Blot analysis Electrophysiology IVF | Case-control | Slo3 knockout mouse | Slo3 knockout mice are infertile. | p < 0.05 |
Table 2: A summary of the studies included for the research question “Can Slo3 be used as potential targets for the modulation of sperm motility and the development of a male contraceptive pill?” in this systematic review. [11,12,15,19,20-23,25,26].
Bioinformatics: Slo1 is also known as calcium-activated potassium channel subunit alpha-1 (KCNMA1). It is located on chromosome 10. In humans, potassium channels are activated by an increase in the cytosolic Ca2+, the concentration of cytosolic Mg2+ and/or membrane depolarization. Potassium channels also aid in the repolarization of the membrane potential. It is highly sensitive to iberiotoxin and charybdotoxin. The kinetics of these channels can be determined by its combination with modulating beta subunits, alternative splicing, and its phosphorylation status. Carbon monoxide bound haem and ethanol increase its channel activation whereas haem inhibits its channel activation Tang et al. [18-23]. Slo3 is also known as calcium-activated potassium channel subunit alpha-3 (KCNU1). We can see that its distribution isn’t that specific to sperm either as it is also present in the brain. The comparison of the human Slo1 channel and the human Slo3 channels shows that they are similar proteins based on their structure (Figure 1). When developing a male contraceptive pill, the specificity of these channels should be considered, focusing on how specific is that channel to sperm and how exclusive is the developed compound to that channel. The blast sequence of all these channels showed that there are some specific similarities as they are all potassium channels but there are also some differences in them as they are different forms of this potassium channel. All sequences are quite similar as they share 95% of sequence morphologies. Based on distribution Slo1 isn’t that specific to sperm. The male contraceptive pill should be developed on the differences of these sequences. There are also 7 different isoforms in humans in their genome, their tissue distribution (Figure 2).
Slo1 study outcomes
Slo1 is insensitive to progesterone, and it is inhibited by alkaline pH Brenker et al. Slo1 has been found present in human sperm by an electrophysiology record conducted in oocytes Leonetti et al. were unable to detect Slo1 in human sperm [24-27]. Slo1 has been reported as the main K+ channel in human sperm. These channels possess high affinity Ca2+ binding sites, indicating that they may represent KSper in humans. The scorpion peptide charybdotoxin (ChTX), IBTX and paxilline block Slo1 channels, suggesting that the Slo1 channel in human sperm is associated with was conducted on the subunits which will control the reaction to these toxins Mannowetz et al. It shows that there is a high expression in the prostate, colon, and the aorta (Expression Atlas, 2021). There is a high expression level in the aorta, which should be considered when developing a male contraceptive. Therefore, we can conclude that Slo1 is not only found in sperm. Based on the expression data available through the Expression Atlas database and the data through the illumines sequencing, we can see that Slo1 is present in twelve different tissues. This could present a huge challenge as separate isoforms mean separate proteins. If these are all in sperm the specificity of the potential male contraceptive may be for different isoforms. The alignment of the Slo1 channels in human vs. mice. We can see that the transmembrane depicted in yellow in both species is the same and the intramembrane depicted in blue is different, with the human one being longer. The data present in the expression atlas is experimental and based on different studies. Further experimental data should be considered on all different locations where Slo1 is present as the data are not clear and there could be potential unknown side-effects.
Slo3 study outcomes
Slo3 has also been found present in human sperm by conducting a western blot analysis López et al. The Slo3 channels are crucial for Ca2+ entering via the CatSper channels during capacitation. Slo3 is activated by alkaline pH Chávez et al. [20]. In humans during capacitation, different K+ channels are required for membrane potential hyperpolarisation. Human sperm is more complex than mice sperm Mansell et al. [22]. Slo3 and the LRRC52 protein are important for male fertility. The knockout LRRC52 males have a lose their fertility potential Zeng et al. Human IKSper pharmacological and biophysical properties correspond to the properties of hSlo3 (human Slo3) but not to the properties of hSlo1 (human Slo1). HSlo3 and IKSper are both activated by Ca2+ and sensitive to pH. Slo3 inhibitors may however block IKSper whereas Slo1 inhibitors do not. hSlo3 is inhibited by progesterone. In mice, sperm Slo3 is the main channel for capacitation induced hyperpolarisation. The mouse Slo3 channel is an alkalisation activated, calcium insensitive potassium channel. Ca2+is the main ligand in hSlo3 whereas H+ is the main ligand in mSlo3 Brenker et al. Slo3-/- male mice are infertile, that could be due to the importance of KSper during cytosolic alkalinisation for the maintenance of a Ca2+ influx via CatSper. A reduced Ca2+ influx may affect the process of hyperactivation which will lead to a compromised fertility Zeng et al.
hSlo3 and mSlo3 show different pharmacological properties. Clofilium does not inhibit hSlo3 but inhibits mSlo3. Quinidine and quinine both block the Slo3 channel in a different potency but by the same site and mechanism. F304Y on the mSlo3 mutation led to a rise in its sensitivity to inhibit quinidine and quinine. This increase in sensitivity cannot be seen in the R196Q mutation. High concentrations of barium led to the blockage of WT mSlo3. F304Y and R196Q mutations lead to an increased channel activity meaning that there is a close function and structure relationship with Slo1 Wrighton et al. [12]. hSlo3-C382R is a naturally occurring variant that has a higher sensitivity to pH and Ca2+ when compared to the hSLO3-WT channels. It is still unknown whether these natural genetic differences will affect male fertility Geng et al. [11].
It depicts the locations where Slo3 is present in the human body. The light blue colour means that the expression levels are low. Slo3 is expressed in the brain and the testes. This may be helpful when making a male contraceptive pill as targeting this channel may not affect other organs or systems to the same extent in the human body. It shows the alignment of the Slo3 channels in humans and mice, differently to what Slo1 showed, the transmembrane depicted in yellow is the same in both species whereas the intramembrane depicted in blue is different. The data present in the expression atlas is experimental and based on different studies. Further experimental data should be considered on all different location where Slo3 is present as the data is not clear and there could be potential unknown side-effects.
Slo1 and Slo3 as targets for male contraception
Slo3 is separated by nonsynonymous substitutions in a higher fixation whereas Slo1 is conserved by a strong purifying selection. Slo3 isoforms are present in several tissues whereas the Slo3 channels are present in mature spermatozoa, spermatocytes and in the testis Chávez et al. [19]. Based on Zhang et al. findings, it seems Slo3 has been evolved to have different functions based on its environment while Slo1 sustains an ancestral function where there is no adaptation. The key differences between the Slo channels are their intracellular gating mechanisms. In hSlo3 there is a Ca2+ sensitivity, which is in a different region compared to the Slo1 sensors. There were various functionally divergent residues identified that had different roles in the Slo1 and Slo3 channels. These residues once identified could explain the differences in function and structure that Slo1 and slo3 have Vicens et al. [18]. There are however similarities in the gating ring structures in both these channels. This implies that there may be a convergence of the Ca2+ and pH mechanisms. The understanding of the way pH affects the functioning of the proteins is difficult, as is can be controlled by various titratable groups. In the Slo3 channels, the molecular identity of their pH sensor is still unknown. The hSlo3 gating ring structure is known, which may aid in further future experiments Leonetti et al. [25]. A comparison of the human and mice biophysical properties shows that in humans, a hyperpolarising K+ current can still pass when the pH is <7.0. The hyperpolarising K+ current in mice was however inactive in those conditions Mansell et al. [22]. There are pharmacological differences between Slo1 and Slo3. A hyperpolarisation was conducted using several K+ channels blockers such as Ba2+, clofilium and TEA+. Ba2+ is a known Slo1 blocker whereas, clofilium and TEA+ are known to block Slo3. Paxilline inhibited Slo1 whereas Slo3 is paxilline insensitive Mannowetz et al. Based on these results, it is noticeable that the Slo3 channels are involved in various mechanisms affecting male fertility making Slo3 a potential target for a male contraceptive pill.
Confounding variables
Slo1 and Slo3 can regulate sperm motility, as demonstrated above, therefore the hypothesis of Slo1 and Slo3 as potential targets for the development of a male contraceptive pill has been proven even though there were inconsistencies in the used studies. Although several scientists have proposed that Slo1 and Slo3 affect sperm motility and fertility the same way they do in mice, there is no consensus on to the extent they affect fertility and whether these channels could be potential pharmacological targets. This research project seeks to establish whether the stated evidence regarding Slo1 and Slo3 as potential targets is adequate, whether there is evidence of the expression of Slo1 and/or Slo3 cellular localisation in human spermatozoa and whether it can lead to the development of a male contraceptive pill. There is a fear of side effects in the male population, this research project will aid in the making of a non-hormonal pill. This research project will not only aid the field of male contraception but will also have a positive impact in the female community as the options of contraception will increase in efficiency. This research will also assist in providing additional information so that it can form the basis of further research to create an effective male contraceptive.
There were inconsistencies regarding whether Slo1 and Slo3 could be potential targets for the modulation of sperm motility and the development of a male contraceptive pill. All studies presented conflicted evidence. One of the main reasons for the present conflicted evidence may be since not all studies used a western blot analysis or a form of cloning and/or electrophysiology to prove the location of Slo1 and Slo3. This was the main argument Brenker et al. had against Mannowetz et al. as Mannowetz et al. stated that Slo1 is present in human spermatozoa whereas Brenker et al. stated that they were unable to detect Slo1 in human spermatozoa. Western blotting, cloning and electrophysiology are crucial steps as the presence of these K+ channels will have to be proved first before finding ways to target them. Another confounding variable in the systematic reviewed studies was the difference in their methodology as studies used a variety of different subjects such as healthy volunteers, mice, sub fertile volunteers, normozoospermic donors, knockout mice, LRRC52 mice, ICSI patients and IVF patients. While the use of different target groups aids us in understanding how Slo1 and Slo3 affects them and the way they can be targeted, there is a variation in the protocols and methodology used to analyse and examine all these groups. It depict a comparison of the alignment sequences of mice and humans. We can see that from a sequence level it looks like they would function the same way as they both take in potassium, but there is not enough evidence that they are regulated the same way. Tables 3-5 show their differences in a physiological level, and we can see that in most cases their functioning and reactions are the exact opposite. In mice, Slo3 and CatSper are voltage and alkaline activated whereas in humans, CatSper is activated by progesterone and prostaglandins. There is also a lower pH sensitivity in human Slo3 and CatSper when compared to mice. Thus, the differences between mice and humans should be taken into consideration when comparing these two target groups. This suggests that the use of mice when developing a male contraceptive pill may not be as representative. An additional repeated issue in this systematic literature review is the lack of inclusion of female factor infertility as in none of the studies reviewed is there a mentioning in their methodology that there were healthy oocytes or healthy female volunteers used, or a way to control that there was no female factor infertility affecting their results. The above factors may have a negative effect on the validity of the presented results.
| Inhibited by paxiline. | Paxilineinsensitive.(Mannowetz et al,2013) |
| Progesterone inhibits Slo1. Thisinhibition will cause depolarization which will open the CatSper (calcium channel) -> raise of Ca2+ -> production of hyperactivation -> allowing sperm to fertilize an oocyte (Mannowetz et al., 2013). | Slo1 is inhibited by alkaline pH. Avdonin et al., (2003) human Slo1is insensitive to progesterone. |
| High affinity Ca2+ binding sites.-> changes In voltage and Ca2+ concentrations (Mannowetz et al., 2013) | Slo3 when there Is a Jack of Ca2+ are sensitive to Intracellular alkalization (Mannowetz et al., (Mannowetz et al., 2013).2013). Slo3 an CatSper are both voltage and alkaline activated (Low CatSperl. |
| Potassium channel blocked by three toxins -> blocks Slo1 (Mannowetz et al., 2013). | Potassium channel blocked by three toxins -> no effect on the Slo3 potassium channels in mice (Mannowatz at al., 2013). |
| Slo1is a major potassium channel in human spermatozoa (Mannowetz et a ., 2013). | It is unknown if Slo3 is important for fertilisation and whether it is functionally expressed in and whether it is functionally expressed in sperm (Brenker et a .,2014). |
| Wang et al 2013 and Brenker et al 2014 could not identify Slo1in human sperm. The mechanicaldifferences between Slo1 and Slo3 are unknown (Vicens ela/.,2019). | Slo3 infertility may arise from this channelbeing needed for acrosome reaction (Zeng et a., 2011) |
Table 3: A comparison of the properties and actions Slo1 and Slo3 channels.
| Human | MouH |
|---|---|
| KSper is inhibited byiberiotoxin, charybdotoxin and paxilline (Mannowetz et al.,2013). | KSper is insensitive to iberiotoxin, charybdotoxin and paxilline (Mannowetz et al.,2013). |
| KSper found either from epididymalor ejaculated spermatozoa were alkalisation independent and sensitive to intracellular calcium (Mannowetz et al.,2013). | The Slo3 channel is an alkalinisat on-activated, calcium-insensitive potassium channel (Mannowetz et al.,2013). |
| This sperm potassium channel comprises the Slo1 protein not Slo3 (Mannowetz et al.,2013). | This sperm potassium channel comprises the Slo3 protein not Slo1 (Mannowetz et al.,2013). |
| It is unclear if the Slo3 channel is present in sperm and whetherit has the same role (Branker et al.,2014). | Slo3 allows potassium ions to flow out of the sperm and make the membrane voltage of these cells negative (Branker et al.,2014). |
| Slo3 is responsible for cell membrane voltage (Branker et al., 2014). | This Slo3 channel opens as a response to a decrease in the concentration of W ions within the sperm (anincrease of the pHinside the cell) (Branker et al., 2014). |
| Changes in calcium ion levels control Slo3. A calcium increase in the cell will open the human Slo3 channel, more than a decrease in the proton concentration does (Brenker et al., 2014). | Slo3 is controlled by pH and cytosolic alkalization (Branker et al., 2014). |
| IKSpet and heterologous expressed Slo3 have similar pharmacology, biophysical properties, and ligand dependence (Brenker et al.,2014). | Deletion of the Slo3 gene stops IKsper (Brenker et al.,2014). |
| The Slo3 pH sensitivity is lower in humans (Brenker et al., 2014). | Slo3 is voltage and alkaline activated (Brenker et al., 2014). Higher Slo3 pH sensitivity. |
| Still unknown whether the pore forming subunit is composed of SL03 and/or SL01(Brown et al.,2019) | A channel involving the pore forming subunit Slo3 and the auxiliary subunit LRRC52 transfers the potassium current (Brown et al.,2019). |
Table 4: A comparison of the Slo1 and Slo3 channels in mice and humans.
| Mutation name | Location | What happens when this channel is inactive? | How does the mutation affect localisation? | Evidence based on. | What was the method for protein detection? | Do they give you any evidence on how they validated the antibody? |
|---|---|---|---|---|---|---|
| Cys-53, Cys-54 and Cys-56 | Intracellular N-terminal S0-S1 linker of BK channels. | Abolishes palmitoylation of BK channels that don’t have the STREX insert. | Decreases localization to the plasma membrane. Eliminates localisation to the plasma membrane. | Jeffries et al, 2010 | Western Blotting, Looked at it on a protein level. | No evidence on antibody validation. |
| R207Q, R207E, R210N AND R213Q | In the S4 domain of the MaxiK hSlo. | It won’t aid with the opening in the absence of Ca2+. | No effect in the coupling between calcium and channel opening. | Diaz et al, 1998 | Electrophysiology | No evidence on antibody validation. |
| T352S | BK Channel | Activated at more negative voltages. Slower rate of inactivation. Impaired inhibition by HMIMP. No effect on channel inhibition by Iberiotoxin. | Gordons et al, 2010 | No evidence on antibody validation. | ||
| S0S1, S0S6, S0S8, S5S8 and S5S10 | In the ion channel C-terminal modulatory domain. | Activated at more negative voltages. No effect on inhibition by HMIMP. | Quirk et al,2001 | Electrophysiology | No evidence on antibody validation. | |
| C615S | Loss of haem-induced channel inhibition. | Tang et al, 2003 | Electrophysiology | No evidence on antibody validation. |
Table 5: A Slo1 mutation table.
Slo1 is highly expressed in the aorta, which should be considered regarding possible effects on circulation when developing a male contraceptive. Slo1 is not only found in sperm, but it is also present in twelve different tissues. This could present a huge challenge as separate isoforms mean separate proteins. If these are all in sperm the specificity of the potential male contraceptive may be for different isoforms. The Slo3 channels are involved in various mechanisms affecting male fertility, making Slo3 a potential target for a male contraceptive pill. More studies are needed to further our understanding of Slo3 and Slo1, as there are a lot of gaps in our knowledge and not enough studies focusing primarily on humans.
I would like to thank my supervisor Dr. Steven Gellatly, who provided me continuous support throughout this project and was there to assist me when needed. I would not have completed this project without his assistance. I would also like to acknowledge my supervisor Professor Christopher Barratt for helping me start off this project.
Citation: Dionelli ME (2021) A Systematic Review Focusing on the Use of Slo1 and Slo3 as Potential Targets for the Modulation of Sperm Motility and the Development of a Male Contraceptive Pill. Andrology. 10:233.
Received: 05-Aug-2021 Accepted: 19-Aug-2021 Published: 26-Aug-2021 , DOI: 10.35248/2167-0250.21.10.233
Copyright: © 2021 Dionelli ME. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.