Journal of Hematology & Thromboembolic Diseases
Open Access
ISSN: 2329-8790
ISSN: 2329-8790
Original Research Article - (2019)Volume 7, Issue 2
This retrospective multi-centred cohort study assessed non-vitamin K oral anticoagulation (NOAC) prescribing in 4 separate general practices in the UK. Using NICE clinical knowledge skills (NICE-CKS) guidelines we assessed the accuracy and validity of NOAC prescribing. We identified 337 patients on NOACs. The most commonly prescribed NOAC was Apixaban (65.3%), followed by Rivaroxaban (20.5%), Dabigatran (10.4%) and Edoxaban (3.8%). Prescribing was predominantly carried out in secondary care (wards 51.9%, clinics 19.3%) with 28.8% of prescribing in general practice. The most common indication requiring anticoagulation was arrhythmias (80.1%), followed by venous thrombus embolus (VTE) (18.1%). The remaining indications were for unlicensed use (1.8%) including left ventricular thrombus, portal vein thrombus, and cardiac transplant. Furthermore, of the patients on a NOAC for an arrhythmia, 2.1% and 0.6% were for atrial tachycardia and recurrent sinus ventricular tachycardia respectively, also unlicensed indications. We found 80 patients (23.7%) on the incorrect NOAC dose with 62% under anti-coagulated 38% over anti-coagulated. Of the patients under anti-coagulated, one had subsequently had a stroke and one a transient ischaemic attack. Of the patients over anti-coagulated, one patient had a significant upper gastrointestinal bleed. The reasons for incorrect prescribing included worsening renal function (64%), deteriorating weight (24%), and increasing age (12%). We found only 30% of patients were followed up adequately at three and twelve months post NOAC prescribing. We found patients that had direct contraindications to NOACs. These contraindications included two patients with an abdominal aortic aneurism, two patients with active cancer and one with a recent haemorrhage.
We also found five patients with provoked VTEs still on a NOAC beyond 6 months treatment. Furthermore, in those patients with unprovoked VTEs requiring lifelong prophylaxis-treatment, we found three patients still on the higher treatment-dose NOAC.
Non-vitamin K oral anticoagulation (NOAC); Tachycardia; Arrhythmia; Renal function
Since 2008 non-vitamin K antagonist oral anticoagulants (NOACs) have been used for the prevention of stroke in patients with nonvalvular atrial fibrillation (NVAF), treatment/secondary prevention of symptomatic venous thromboembolism (VTE) and prevention of VTE after major orthopaedic surgery.
There are numerous perceived benefits to NOAC prescribing over warfarin. It is well accepted that warfarin suffers from numerous commonly used drug and food interactions resulting in labile international normalized ratios (INRs). In addition, the fixed dosing of NOAC prescribing, without the need for regular, often monthly INR blood tests, make NOACs an attractive alternative to warfarin when anticoagulation is clinically indicated.
In the UK, the rate of initiation of NOACs has increased substantially since 2008 and these agents have now surpassed warfarin as the anticoagulant of choice [1]. However, for all their perceived ease of use, numerous studies indicate that NOAC prescribing falls short with regard to accurate dosing, indications for NOAC use and direct contraindications [2-5]. At present four NOACs, Apixaban, Edoxaban, Rivaroxaban (factor X inhibitors), and Dabigatran (thrombin inhibitor) are licensed for use as anticoagulants in the UK.
NICE-CKS guidelines for NOAC prescribing were last revised in 2016 (https://cks.nice.org.uk/anticoagulation-oral). Although regular monitoring is not recommended, in light of the renal/ hepatic excretion of NOACs, initial blood tests should be performed for U&Es, eGFR, LFTs and clotting. Furthermore, a three-month review is recommended to monitor patients for side effects and drug compliance. In addition, annual blood tests should be performed on patients prescribed NOACs, or more frequently if the patient falls ill. Specific criteria on renal function and NOAC prescribing also exists, with NOAC dose changes being required with deteriorating creatinine clearance. Finally, age is also a factor when prescribing NOACs.
The potential harm caused by inaccurate anticoagulant prescribing is considerable. This retrospective multi-centred cohort study assessed NOAC prescribing in four separate general practices covering over 40,000 patients. Using NICE-CKS guidelines as our standard, we assessed the appropriateness of NOAC prescribing with respect to dosing, indication/contraindication and longevity of treatment.
Using System One we identified patients prescribed NOACs by applying search criteria for patients who have NOACs on their repeat prescriptions. This retrospective cohort study identified 337 patients currently being prescribed NOACs in four general practices. We assessed the appropriateness of NOAC prescribing using NICE-CKS guidelines as our standard.
Of the 337 patients prescribed NOACs identified in our study, the most commonly prescribed NOAC was Apixaban (65.3%), followed by Rivaroxaban (20.5%), Dabigatran (10.4%) and Edoxaban (3.8%) (Figure 1). Further analysis of NOAC prescribing showed that initiation of prescribing was predominantly carried out in secondary care (hospital wards 51.9%, clinic 19.3%). With 28.8% of prescribing initiated in general practice (Figure 2).
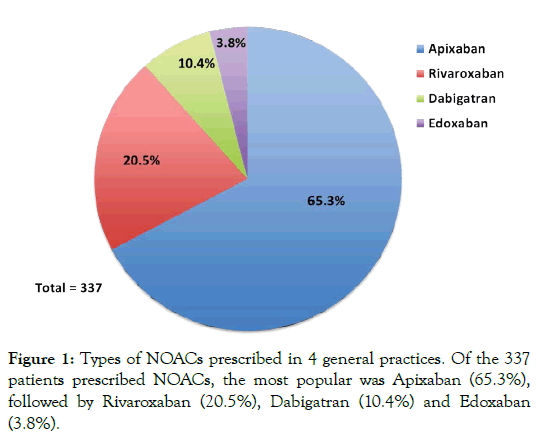
Figure 1: Types of NOACs prescribed in 4 general practices. Of the 337 patients prescribed NOACs, the most popular was Apixaban (65.3%), followed by Rivaroxaban (20.5%), Dabigatran (10.4%) and Edoxaban (3.8%).
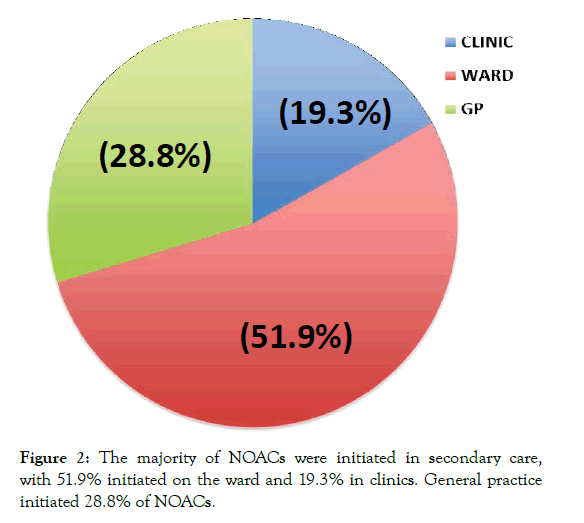
Figure 2: The majority of NOACs were initiated in secondary care, with 51.9% initiated on the ward and 19.3% in clinics. General practice initiated 28.8% of NOACs.
NOACs are licenced for use for stroke prevention in patients with NVAF, treatment and prevention of VTE disease and VTE prophylaxis post surgery. We investigated the indications for NOAC prescribing within four general practices. The most common indication was for arrhythmias 80.1%, followed by VTE with 18.1%. The remaining indications were for unlicensed disorders, 1.8% (Figure 3). Unlicensed indications included four patients prescribed NOACs for left ventricular thrombus, one patient with a portal vein thrombus, and one patient with a cardiac transplant. Of the 80.1% of patients prescribed NOACs for arrhythmias, 96.4% of these were for atrial fibrillation/paroxysmal atrial fibrillation (AF/ pAF), 2.4% were for atrial tachycardia (AT) and 1.2% for recurrent sinus ventricular tachycardia (SVT). Both AT and SVT are further unlicensed indications for NOAC prescribing.
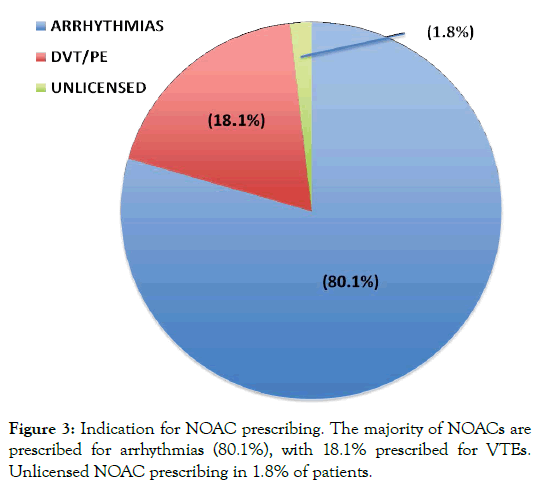
Figure 3: Indication for NOAC prescribing. The majority of NOACs are prescribed for arrhythmias (80.1%), with 18.1% prescribed for VTEs. Unlicensed NOAC prescribing in 1.8% of patients.
Of the 337 patients identified in this study, 80 patients were found to be on the incorrect NOAC dose (23.7%). Of these, 50 patients (62%) were under anti-coagulated and 38% over anti-coagulated (Figure 4). Further analysis looking at individual NOACs 26% of patients on Apixaban and Rivaroxaban, 39% patients on Dabigatran, and 9% of patients on Edoxaban were on the incorrect dose (Figure 5). The reasons found for incorrect dosing included worsening renal function 64%, declining weight 24%, increasing age 12% (Figure 6). Of those patients under anti-coagulated, one patient had a stroke, and one patient had a transient ischaemic attack. Furthermore, of the patients over anti-coagulated, on patient had an upper gastrointestinal bleed.
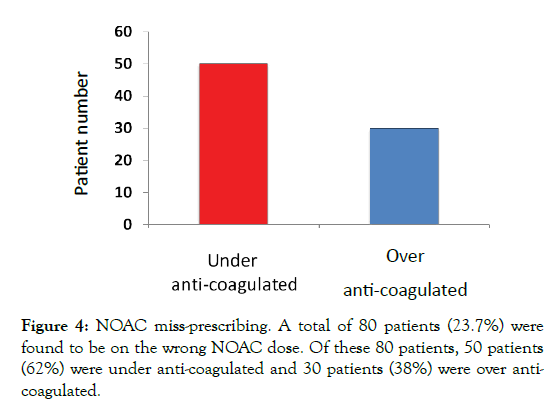
Figure 4: NOAC miss-prescribing. A total of 80 patients (23.7%) were found to be on the wrong NOAC dose. Of these 80 patients, 50 patients (62%) were under anti-coagulated and 30 patients (38%) were over anticoagulated.
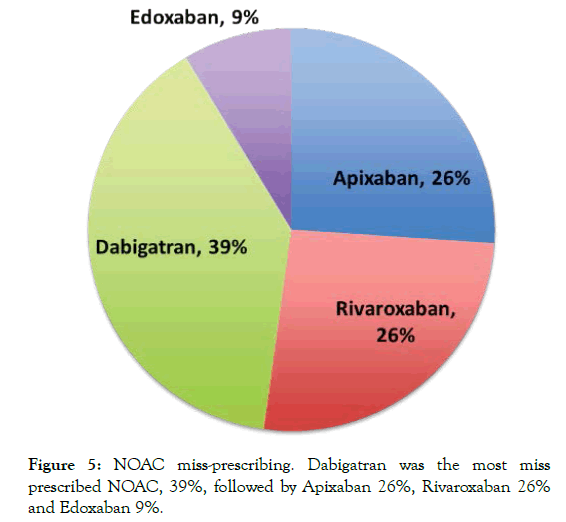
Figure 5: NOAC miss-prescribing. Dabigatran was the most miss prescribed NOAC, 39%, followed by Apixaban 26%, Rivaroxaban 26% and Edoxaban 9%.
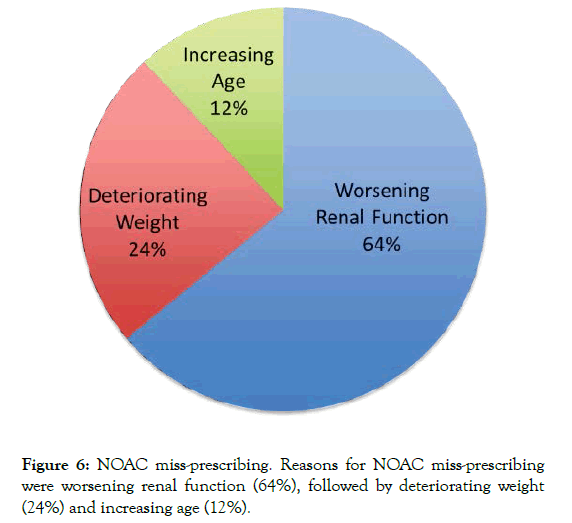
Figure 6: NOAC miss-prescribing. Reasons for NOAC miss-prescribing were worsening renal function (64%), followed by deteriorating weight (24%) and increasing age (12%).
NICE-CKS guidance on NOAC prescribing recommends 3 months follow up to check drug compliance and side effects, and 12 months follow ups to assess organ function. We found that only 30% of patients prescribed NOACs had adequate follow up at both 3 and 12 months (Figure 7).
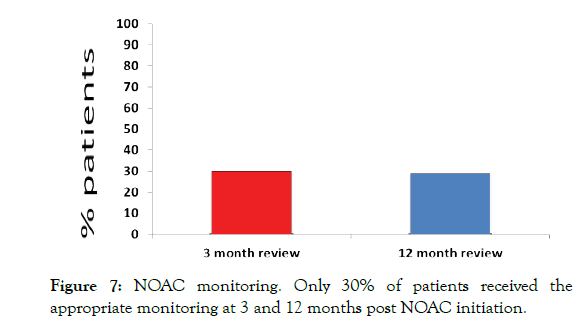
Figure 7: NOAC monitoring. Only 30% of patients received the appropriate monitoring at 3 and 12 months post NOAC initiation.
Various contraindications to NOAC prescribing exist in NICECKS guidance. We found 5 patients who had contraindications to NOAC use, these included two patients with an abdominal aortic aneurism (AAA), two patients were found to have active cancer, and one with a recent haemorrhage (Figure 8).
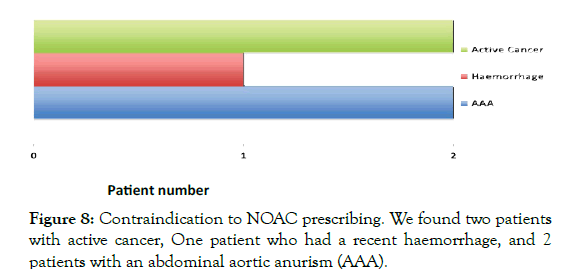
Figure 8: Contraindication to NOAC prescribing. We found two patients with active cancer, One patient who had a recent haemorrhage, and 2 patients with an abdominal aortic anurism (AAA).
Provoked VTE guidelines indicate NOACs should be stopped after 3-6 months of treatment. We found 5 patients who were still taking NOACs beyond 6 months for provoked VTEs (Figure 9). Furthermore, according to NICE-CKS guidance those patients requiring long term anticoagulation for unprovoked VTEs, patients should be switched from NOAC treatment-dosing to NOAC prophylaxis-dosing after 6 months treatment. We found 3 patients still on NOAC treatment-dosing who had not been switched to the lower prophylaxis-dosing (Figure 10).
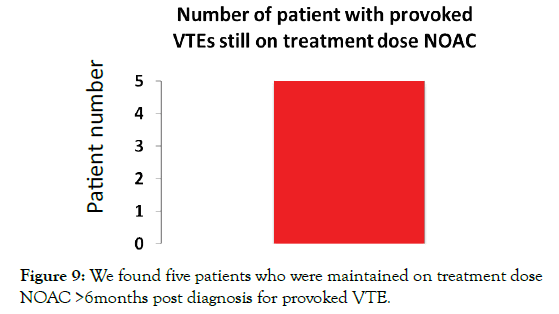
Figure 9: We found five patients who were maintained on treatment dose NOAC >6months post diagnosis for provoked VTE.
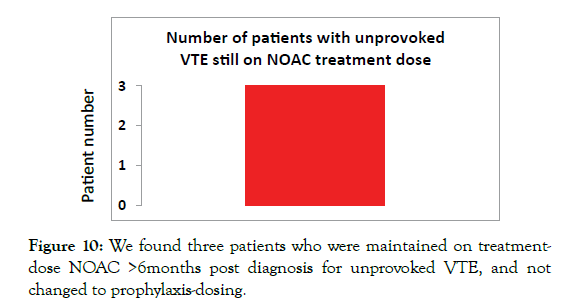
Figure 10:We found three patients who were maintained on treatmentdose NOAC >6months post diagnosis for unprovoked VTE, and not changed to prophylaxis-dosing.
Non-vitamin K oral anticoagulants (NOACs) are now in their 10th year of prescribing in the UK. From their introduction, much was made of their ease of use, with no need for regular blood tests to monitor the INR. Furthermore, with warfarin interacting with so many commonly used medications, resulting in labile INRs, NOACs are now the drug of choice when considering anticoagulation for stroke prevention in patients with NVAF, treatment and prophylaxis management of VTE and VTE prevention post surgery [1]. However, studies indicate that the accuracy of NOAC prescribing is falling short of local prescribing guidelines. Initial studies looking at the original ARISTOTALE trial and the SPRINT-AF registry pointed toward the under-dosing of NAOCs [6,7].
Real-world studies investigating NOAC prescribing continue with this theme, that NOACs are being miss-prescribed to patients. One study looking at 198 patients attending an emergency department identified 16.7% of patients prescribed NOACs were on the incorrect dose according to local guidelines [2]. A multi-centred cohort study with 167 subjects, encompassing three teaching hospitals identified the level of incorrect NOAC prescribing at 34%, with the most common cause of inaccurate prescribing in this study due to deteriorating renal function [3]. A large retrospective cohort study encompassing over 6000 patients identified inaccurate prescribing in 7.7% of patients [5,8-10].
Here we undertook a retrospective cohort multi-centred study assessing NOAC prescribing in four general practises in the UK. We identified 337 patients on NOACs. We found that the most commonly prescribed NOAC was Apixaban (65.3%), which compared to the other NOACs has a stricter prescribing protocol, requiring renal function, weight and age assessment prior to prescribing (NICE-CKS guidelines). We show that 23.7% of patients identified as being prescribed NOACs, were on the wrong dose, with the most common NOAC to be miss-prescribed being Dabigatran (39%).
In the cohort of patients under prescribed NOACs, one patient subsequently had a stroke, with another suffering a transient ischaemic attack. Furthermore, in the over-prescribed cohort one patent had suffered an upper gastrointestinal bleed resulting in a considerable drop in haemaglobin to 70 dl/l. This data is in keeping with previous studies, which highlight the potential harm when oral anti-coagulants are either under or over prescribed [2].
A common theme to miss-prescribing anticoagulant medications is that under prescribing is more prevalent than over prescribing [5,11,12]. Here we show that there is a tendency to under prescribe relative to over prescribing at a ratio of 1.65:1. It is thought that this trend of under prescribing is due to clinicians being more cautious over bleeding risk, than stroke and VTE risk when prescribing anticoagulation medications.
Similar to previous studies [3], the most common cause for missprescribing in our study was deteriorating renal function (64%). This is no surprise considering all NOAC prescribing is renal function dependent, requiring a reduction in dosing if creatinine clearance (CrCl) falls below 49 ml/min for Rivaroxaban, 50 ml/ min for Edoxaban and Dabigatran and 29 ml/min for Apixaban. Further indications for NOAC dose reductions include patient age (>80yrs) for Dabigatran and Apixaban, with age accounting for 12% of miss-prescribing. Finally, Apixaban dose reductions also account for patient weight (<60Kg), which accounts for 24% of miss-prescribing.
Furthermore, previous studies have identified an issue with follow up post NOAC prescribing. One study with recommendations to review at 1 month and 12-month post prescribing suggested that a prescribing review occurred only 39% and 43% of the time respectively [4]. NICE-CKS guidelines recommend a review 3 months and 12 months post prescribing. Here we show that these reviews were only occurring 30% of the time, which may go some way to account for the prescribing inaccuracy of 23.7%.
In summary, although guidelines exist for NOAC prescribing, we and others have found that the accuracy of prescribing, when compared to local guidelines is falling short. Here we show that 23.7% of patients are on the wrong NOAC dose.
Recent studies looking at methods to improve NOAC prescribing accuracy, implemented pharmacy screening prior to patient hospital discharge. However, these studies only showed modest improvement in prescribing accuracy [12,13].
In some areas of the UK, NOAC prescribing and monitoring has been transferred to local anticoagulation clinics, which although may improve prescribing accuracy, increases costs for cash strapped CCGs. Recently, in general practice, the local CCG have provided a useful tool on System One with a clear protocol of choice of NOACs when anticoagulation is clinically indicated. Within this tool general practitioners are able to prescribe NOACs safely, with weight, age and cretinine clearance taken into consideration before allowing prescriptions to be issued. However, no such protocol exists in secondary care where the majority of NOAC prescribing takes place.
Citation: Smith JR, Barhey M (2019) Accuracy of NOAC Prescribing in Primary and Secondary Care: Retrospective Cohort Study J Hematol Thrombo Dis 7: 303, doi: 10.35248/2329-8790.19.07.303
Received: 25-Jul-2019 Accepted: 15-Oct-2019 Published: 22-Oct-2019
Copyright: © 2019 Smith JR, et al. This is an oepn access article distributed under the terms of the creative commons attribution license, which permits unrestricted use, distribution, and reproduction in any medium,provided the original work properly cited.