Advances in dairy Research
Open Access
ISSN: 2329-888X
ISSN: 2329-888X
Research Article - (2013) Volume 1, Issue 1
In this study, cheddar cheeses made with adjunct cultures L. casei ssp. casei 300 (CCA) and L. paracasei ssp paracasei 22 (CCB) were assessed for ACE inhibitory activity at different stages of ripening. ACE-inhibitory activity of the UF permeates of WSE of all the cheeses increased significantly (P<0.05), especially after the first two months of ripening. The inhibitory activity continued to increase in the cheeses made with adjunct cultures CCA (IC50 value 0.16 mg.ml-1) and CCB (IC50 value 0.20 mg.ml-1) up to third month and in CCC increased to this level after fourth month of ripening (IC50 value 0.20 mg.ml-1). The electrophoretic and RP-HPLC profiles indicated that the rate of degradation of proteins resulted in formation of smaller peptides which were higher in cheese made with adjunct cultures as compared to control cheese The result also indicated that cheeses with the addition of adjunct had higher ACE-inhibitory activity than control cheese.
Keywords: Cow milk, SDS-PAGE, Water soluble extracts, RP-HPLC analysis, Inhibitory activity
The benefits of the fermented dairy products in the diet are well accepted. The central role of microorganisms in fermentation, especially Lactic Acid Bacteria (LAB) is now widely acknowledged, and it is accepted that these microorganisms can exert beneficial effects through two mechanisms: direct effects or indirect effects during fermentation where these microbes act as cell factories for the generation of secondary metabolites with health promoting properties. Among the latter the most important components in fermented milk are bioactive peptides released from milk proteins by members of the genera Lactobacillus, Bifidobacterium and other LAB. There are few reports on the bioactive peptides release in cheeses during ripening [1-5]. Cheese contains phosphopeptides as natural constituents and extensive proteolysis during cheese ripening leads to the formation of other bioactive peptides such as ACE-inhibitory, antioxidant, antimicrobial and immunomodulatory. ACE-inhibitory peptides inhibit the activity of Angiotensin I converting enzyme (ACE) which catalyses the hydrolysis of the inactive prohormone angiotensin I (decapeptide) to angiotensin II (octapeptide) that increases blood pressure through vasoconstriction. ACE also inactivates the vasodilating peptide bradykinin (nonapeptide) and endogenous opioid peptide Met-enkephalin. ACE inhibitory peptides have been separated from several Italian cheese characterized by short (Crescenza and Italico) and medium (Ghorgonzola) ripening period [5]. The new Fesitvo cheese based on an innovative concept in the production of healthy foods has been commercially developed [4]. It has been reported that the ACE-inhibitory peptides increases during ‘Fesitvo’ cheese ripening and decreases when proteolysis exceeds a certain level during the storage period. Pripp et al. [6] have investigated the relationship between proteolysis and ACE inhibition in Gamalost, Castello, Brie, Pultost, Norvegia, Port Salut and Kesam and their results also indicate an increase in ACE inhibitor activity during the course of cheese ripening. These results suggested the ACE inhibitory peptide and probably other biologically active peptides are naturally found in cheese. The water soluble extracts of cheese contain major peptides formed during ripening [7]. The main objective of the present study was to evaluate the ACE inhibitory activity of water soluble extracts of Cheddar cheeses made with and without adjunct cultures at different stages of ripening and its correlation with extent of proteolysis.
Materials
Cow milk was obtained from the Experimental dairy, National Dairy Research Institute (NDRI) Karnal, India to prepare cheddar cheese. The cheese culture NCDC No CH-149 and Lactobacillus casei ssp. casei 300 and Lactobacillus paracasei ssp. paracasei 22 were procured from National Collection of Dairy cultures (NCDC) at NDRI, Karnal. Microbial rennet (Meito rennet) was supplied by Meito Sangyo Co. Ltd (Nagoyan, Japan). The chemical Hippuryl-L-histidyl-LLeucine (HHL). Angiotensin converting enzyme (ACE) were procured from Sigma-Aldrich (St. Louis,Mo, USA). All other reagents used were of analytical grade procued from SRL (New Delhi, India and qualigens Mumbai, India).
Manufacture of cheddar cheese
The cow milk was standardised with fresh cow cream in order to obtain casein to fat ratio of 0.7 and pasteurized. The cheeses were manufactured according to the standard procedures of Kosikowski as modified by Kanawji [8]. Cheddar cheese culture (NCDC-149) was added to cheese milk at rate of 1% (v/v) to prepare control cheddar cheese (CCC), NCDC CH-149 (1%) along with 0.5% (v/v) adjunct culture L. casei ssp casei 300 for cheddar cheese A (CCA) and NCDC CH-149(1%) along with 0.5% (v/v) adjunct culture L. paracasei ssp paracasei 22 for cheddar cheese B (CCB). After a 0.02% increase in the acidity of milk over the initial value (about 40 min after starter addition), rennet 1.5 g/100l milk was added to milk. All the three batches i.e., CCC, CCA and CCB were produced from the same pasteurized cow milk. Three replicates of experimental (CCC, CCA and CCB) and control cheddar cheese were made and analysed for the extent of proteolysis and ACE inhibitory activity.
Preparation of water soluble extracts (WSEs)
Water soluble extracts of the peptides formed in cheddar cheese (CCC, CCA and CCB) were prepared using the method developed by Kuchroo and Fox [7]. 20 g of grated cheddar cheese was mixed with 60 ml of glass distilled water and sonicated (Ultrasonicator, Sonics Model VC 750, vibra cell, Newton LT, USA). The mixtures were centrifuged at 14000 g for 10 min in refrigerated centrifuge (Heraeus, centrifuge, Hanan, Germany). The fat layers were removed and the water extracts were filtered through whatman No.1 filter paper. The pH of the extracts was adjusted to 4.6 using 1N HCl. The precipitated proteins were removed by filtering through whatman No.1 filter paper. To further remove any impurities, the water soluble extracts were filtered through 0.22 μm pore size filter (Millipore, Billerica, MA, USA). Water soluble extracts of cheddar cheese was subjected to ultrafiltration (UF) using Pall stirred Cell UF system (150 ml capacity, omega cell, 5 kDa cut off membrane), under nitrogen pressure of 50 psi. Permeate of the samples were collected and evaluated for protein content according to the method of Lowery et al. [9].
SDS-PAGE of Water Soluble extracts (WSEs) of cheese samples
The extent of proteolysis of cheese samples was assessed using tricine SDS-PAGE performed according Schagger and Von Jagow [10] method on 16.5% slab gel (separating gel) with slight modification. Samples were prepared by mixing water soluble extracts of cheddar cheese of different ripening stages with equal volume of sample buffer (pH 6.8). The vertical electrophoresis unit (BioRad Midi) was used and the samples were run at 25 mA for twelve hours. The gels were stained using Coomasie Blue R-250 (0.23%) and the detected polypeptides were identified using low molecular weight kit having proteins of 66.0, 43.0, 29.0, 18.4 and 6.5 kDa (Banglore Genei).
RP-HPLC analysis of water soluble extracts (WSES) of cheese samples
The RP-HPLC system (Shimadzu Kyoto, Japan) was used for the profiling of water soluble peptides of the permeate of the water soluble extracts of the cheese samples. The permeate was treated with 0.1% trifluoroacetic acid (TFA) and supernatant was collected. The 20 μl of supernatant containing TFA soluble peptides was injected on to a RP-HPLC column (Shimpak C18, AG 120 A, 5μm). The elution was performed at 0.5 ml min-1 flow rate with eluent A (0.1% TFA in water) and eluent B(0.1% TFA and 90% Acetonitrile in water) using linear gradient: 0 to 32% B for 20 min, followed by 32-47% B for 20-80 min. The separated peptides were monitored at 215 nm using UV detector.
Angiotensin Converting Enzyme (ACE) inhibition assay
Angiotensin converting enzyme (ACE) inhibitory activity was measured using the method of Cushman and Cheung [11]as modified by Hernandez et al. [12].The method is based on the liberation of hippuric acid from hippuryl-L-histidyl-L-leucine (HHL) catalyzed by ACE. 20 μl of sample was added to 110 μl of substrate (5 mM HHL in 0.1 M borate buffer containing 0.3 M NaCl, pH 8.3). After addition of 20 μl ACE (4 mU), the reaction mixture was incubated at 37°C for 30 min. The reaction was terminated by the addition of 250 μl of 1M HCl. The hippuric acid formed was extracted with 1.5 mL of ethyl acetate (by centrifugation at 3000 g for 10 min). An aliquot of one mL of the upper organic layer was collected and dried out completely by heating at 95°C for 20 min, redissolved in 1 mL distilled water and measured spectrophotometrically at 228 nm. The activity of each sample was tested in triplicate. The positive control of the reaction was carried out by adding only substrate, ACE and water (no sample). The blank was prepared by only substrate and water (ACE volume was replaced by equal amount of water).
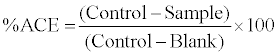 (1)
(1)
Results were expressed as peptide concentration required inhibiting 50 percent of the original ACE activity (IC50).
SDS-PAGE and RP-HPLC Analysis of WSEs of Cheddar Cheeses
The compositional and sensory analysis of these cheese samples were presented in our earlier study [1]. The Tricine- SDS PAGE of water soluble extracts of all the three cheeses (CCC, CCA and CCB) was performed to see the extent of proteolysis with ripening time from 0 to 9 months and electrophoretic pattern were presented in figure 1 (Plates 1-3). The number of lower molecular weight bands (<6500) were less in the early ripening period of all the cheeses, increased during 4 to 6 months and further decreased during 7 to 9 months in all the cheeses. The cheeses made with adjunct cultures (CCA and CCB) indicated higher number of bands. From the electrophoretic pattern it has been assumed that the rate of proteolysis reached to its maximum level between 4 to 6 months and then decreased for further ripening.
Figure 1: Tricine-PAGE pattern of WSE of Control Cheddar Cheese (Plate 1) from 1 to 9 months (1-9 lane and lane 10 Markers), Cheddar cheese made with L. casei ssp. casei (Plate 2) from 1 to 9 months (2-10 lane and lane 1 Markers) and Cheddar cheese made with L. paracasei ssp. paracasei (Plate 3) from 1 to 9 months (1-9 lane and lane 10 Markers).
The RP-HPLC chromatogram of UF permeates of water soluble extracts CCC, CCA and CCB at different stages of ripening were shown in figures 2-4. It can be inferred that as the age of cheese increased, new peaks appeared while existing peaks at initial stages of ripening either increased or decreased. Main peaks were observed with elution times starting from 6 to 55 mins. The rate of formation of peptides in the cheeses made with adjunct CCA and CCB cultures were higher as Compared to Control Cheese (CCC). The protein content in water soluble extracts and electrophorograms (Plates 1-3) also indicated that rate of proteolysis was high in case of CCA and CCB during early stages of ripening. Similar to previous findings for full fat cheddar cheese [13,14], chromatograms for all the cheeses had a large number of peaks, indicating a heterogeneous mixture of proteolysis products in UF permeates of water soluble extracts of cheddar cheeses. It has been noted that the elution times of peptides with higher retention times of peptides are often affected by their average hydrophobicity on the reverse phase column [15]. Higher absorbance values of peaks indicate higher concentration of peptide contents present in UF permeates of water soluble extracts of cheddar cheeses. With the increase in ripening time from 4 to 6 months the electrophoretic pattern and elution profile remained almost similar. Further increase in ripening time lead to decrease in number of bands in the electrophorograms (Plates 1-3) as well as number of peaks in RP-HPLC elution profiles (Figures 2-4).
McSweeney [16] summarized the pattern of proteolysis in many varieties of cheeses which indicates that the caseins are hydrolyzed initially by residual coagulant activity retained in the curd and by plasmin (and perhaps other indigenous proteolytic enzymes) to a range of large and intermediate sized peptides that are hydrolysed by proteinases and peptidases from LAB and NSLAB and perhaps secondary micro-flora to shorter peptides and amino acids.
Angiotensin converting enzyme inhibitory activity of UF permeates (<5 Kda) Of WSEs of cheddar cheeses
The ACE-inhibitory activity of ultrafiltered (UF) permeates (<5 kDa) of WSEs of cheese samples (Control cheese (CCC), cheddar cheese made with adjunct cultures L. casei ssp. casei 300 (CCA) and L. paracasei ssp. paracasei 401 (CCB)) collected at one month interval up to nine months was compared by determining the amount of protein needed to inhibit 50% of original ACE activity (IC50) and is shown in table 1. ACE-inhibitory activity of the UF permeates of WSE of all the cheeses increased significantly (P<0.05) especially during the first two months of ripening (Table 1). The inhibitory activity continued to increase in the cheeses made with adjunct cultures CCA and CCB up to third month and in CCC increased up to fourth month of ripening. The IC50 of the CCA (0.16 mg.ml-1) and CCB (0.20 mg.ml-1) at the end of third month was significantly lower (P<0.05) than that of the control cheese (0.29 mg.ml-1). In control cheese the lowest IC50 value was attained after fourth month (0.20 mg.ml-1). The ACE inhibitory activity of all the cheeses decreased after fourth month of ripening in the cheeses made with adjunct cultures and after sixth month in the control cheese. The result indicated that cheeses with the addition of adjunct had higher ACE-inhibitory activity during the third month of ripening than control cheese, but after fourth month of ripening the control cheese showed better ACE inhibitory activity. On further ripening overall decrease in ACE-inhibitory activity with the increase in IC50 was observed in all the cheese samples (Table 1). ACE inhibitory activity increased with increase in the protein content of the WSE of cheddar cheeses as depicted from the electrophorogram and RPHPLC profiles of cheese samples (Figures 1-4). This indicates the soluble protein (peptides) increases in the WSEs which may contribute toward ACE inhibitory activity. Similarly in the another study form our research group showed that increased protein content in WSEs of during cheeses ripening related to the proteolysis in the cheeses [1]. Similar results were obtained by other workers which indicate that the activity increases as the proteolysis proceeds but to a certain extent. The presence of active peptides that are naturally formed in cheese depends on a delicate equilibrium between their formation and their degradation by the proteolytic systems involved in cheese ripening. ACE inhibitory peptides have been found in several types of cheeses, which differ with respect to the type of starter and the ripening condition used [4,17-19]. Ong et al. [2] developed cheddar cheese manufactured with starter Lactococci and Lactobacillus casei. They found that the IC50 (concentrations of ACE inhibitors needed to inhibit 50% of ACE activity) was the lowest after 24 weeks of ripening in the probiotic cheeses (0.23-0.25 mg.mL-1) compared to 36 weeks for cheeses without any probiotic (0.28 mg.mL-1) which were slightly higher than reported IC50 value in the present study.
| Ripening Months | CCC | CCA | CCB |
|---|---|---|---|
| Mean ± SEM | Mean ± SEM | Mean ± SEM | |
| 0 | 1.579 ± 0.004 | 1.674 ± 0.010 | 3.006 ± 0.078 |
| 1 | 1.958 ± 0.025 | 1.794 ± 0.002 | 2.624 ± 0.058 |
| 2 | 0.283 ± 0.003 | 0.165 ± 0.001 | 0.261 ± 0.004 |
| 3 | 0.293 ± 0.012 | 0.160 ± 0.002 | 0.208 ± 0.011 |
| 4 | 0.202 ± 0.003 | 0.204 ± 0.001 | 0.561 ± 0.031 |
| 5 | 0.225 ± 0.009 | 0.243 ± 0.000 | 0.584 ± 0.013 |
| 6 | 0.206 ± 0.004 | 0.375 ± 0.015 | 0.572 ± 0.006 |
| 7 | 0.249 ± 0.003 | 0.347 ± 0.012 | 0.564 ± 0.004 |
| 8 | 0.286 ± 0.004 | 0.351 ± 0.015 | 0.555 ± 0.008 |
| 9 | 0.251 ± 0.002 | 0.399 ± 0.004 | 0.662 ± 0.006 |
ACE inhibitory activity is expressed as IC50 value (as the amount of protein content needed to inhibit 50% of the ACE inhibitors). Each observation is mean of three replicates of each batch. Results are expressed as mean of scores ± standard error of mean.
Table 1: ACE-inhibitory activity (IC50 value mg.mL-1) of UF permeates (<5 kDa) of WSEs of Cheddar cheeses (CCC, CCA and CCB) during whole ripening period.
Meisel et al. [20] reported that ACE inhibition in Cheddar cheeses was dependent on proteolysis to a certain extent. The ACE-inhibitory activity in medium aged Gouda was about double that of long ripened gauda. A αs-casein derived peptide isolated from 6 month ripened Parmesan cheese was not detectable after 15 months of ripening. Products having a low level of proteolysis (Quarg) have a low ACE inhibition index. These results indicate that the bioactive peptides liberated by proteolytic enzymes from LAB during cheese ripening were degraded further to inactive fragments as a result of further proteolysis [20]. Gomez and his co-workers 2002 observed that 15 days old Manchego cheese showed low ACE-inhibitory activity; this manchego cheese was prepared from ovine milk. Furthermore, this inhibitory activity decreased during the first 4 months, increased when proteolysis advanced, and decreased again in 12-month-old cheese. Ryhanen et al. [4] showed that the ACE inhibitory activity of a fermented low fat hard cheese produced with probiotic lactic acid bacteria increased during cheese ripening and decreased when proteolysis exceeded a certain level. These ACE inhibitory peptides are derived from αs1-casein which emerged at the age of three months and their levels remain stable for six months. Similar trends were seen in the present study (Table 1) and Similary, Pritchard et al. [3] indicated that commercial cheese sample which was matured for longest time, highest ACE inhibitory activity due to production of more ACE inhibitory peptides.
ACE-inhibitory activity in the cheeses can be enhanced by using the highly proteolytic strains of LAB. But the technological intervention is to choose the right strains or combination of strains with optimal proteolytic activity for such dairy products. The strains should not be too proteolytic to destroy the product but should be able to produce the bioactive peptides with desired activities. Moreover the content of potent peptides depends on a balance between their formations and further breakdown into inactive peptides and amino acids, during ripening of the cheeses.