Journal of Drug Metabolism & Toxicology
Open Access
ISSN: 2157-7609
ISSN: 2157-7609
Research Article - (2012) Volume 0, Issue 0
The present study was carried out to assess the toxicological effect of acetamiprid, an insecticide of neonicotinoid group on clinical and serum hematobiochemical parameters of mice. The experimental animals were divided into four different groups of equal number of animals and the first group was considered as Control. It was found that acetamiprid administered @ 40 mg/kg body weight per day (1/5th of LD% value) for a period of 28 days induces prominent toxic symptoms along with hematological and biochemical effect in mice. Acetamiprid induced mice showed decrease in body weight and clinical symptoms like respiratory depression, sitting in the corner, diarrhea, depression, dullness etc. are observed at the different dose levels. Test mice administered with acetamiprid showed significant decrease in overall hematological profile. In the present study, it was found that acetamiprid @ 10 mg/
kg body weight (1/20th of LD50 value) did not produce significant changes in hematological and biochemical values. So, it was considered that experimental dose of 10 mg/kg to be non-toxic and 40 mg/kg body weight to be the toxic dose of acetamiprid in mice.
Keywords: Acetamiprid; Clinical; Hematobiochemical; LD50 value
Acetamiprid, a member of the neonicotinoid group of insecticide is highly effective for the controlling aphids, beetles, moth, leafhopper, pests on crops and leafy vegetables, along with fleas infesting livestock and pet animals. It is a systemic insecticide with translaminar action which has a contact and stomach action. Moreover, acetamiprid being highly water soluble indicates a high potential for the compound to leach in soil or to run off in surface water. Acetamiprid is the most highly effective and largest selling group of insecticides worldwide for crop protection [1]. Therefore, the relative risks and benefits of this insecticide must be compared to existing pesticide. Although, acetamiprid is highly used in India and abroad but there are still many doubts related to its toxicity and health hazards.
Exposure of animals and birds to insecticides for a short duration induces a state of stress leading to decrease in production and behavioral as well as biochemical changes [2]. The continuous use of pesticide imposes hazardous effect on the physiological function of various body systems. The man and animal exposed to low level of pesticides residues in air, water, and food chain.
The need for higher agricultural production as one of the prerequisites for improving the population’s standard of living involves the use of various chemicals. Pesticides to prevent losses of cultivated plant, food and feedstuff stress.
However, large scale use of pesticides has brought about many problems. In this way thousands of tons of chemical compounds often very toxic or with other properties are in current use.
It is in view of paucity of information available on acetamiprid toxicity regarding its biochemical alterations in mice, the present study was designed on induced acetamiprid toxicity at different dose levels. It has been planned on mice as a model animal with the objectives of studying the clinical manifestation of acetamiprid toxicity in mice, to evaluate the hematological values at different dose levels and to analyze the hematobiochemical profile.
Chemicals
Acetamiprid,(E)-N’-[(6-chloro-3-pyridyl)methyl]-N2-Cyano-N1- Methylacetamidine (IUPAC), 95% Pure (20%SP).
Experimental animals
Sexually matured 80 No. mice of average 45 days old of either sex weighing 20-25 g were bred in laboratory animal facility affiliated to Department of Veterinary Pathology, West Bengal University of Animal and Fishery Sciences, Kolkata, India. All breeding phases and all experiments were performed in accordance with the rules of the “Committee for the purpose of control and supervision on experiments on animals”. All animals were housed in galvanized cages with paddy husk bedding. Animals were maintained under standard conditions of feeding and management.
Group II to IV served as treatment Groups and Group I served as control Group in this sub-acute toxicity study. Group I (control group) was administered with vehicle i.e. distilled water @10 ml/kg of dose volume. Dose volume was same for all treatment Groups. The LD50 Value of acetamiprid (95% pure, 20% SP) was reported to be 198 mg/ kg (approx.200 mg/kg) in male mice and 184 mg/kg in female mice by manufacturers (Nanjing AGG Chemical Co. Ltd.). Considering this LD 50 value, three different dose levels selected i.e. 1/20th (10 mg/kg), 1/10th (20 mg/kg), 1/5th (40 mg/kg) of the reported LD 50 value were employed in the present study (Table 1). The mice of Group II, III and IV were administered 10 mg/kg, 20 mg/kg, and 40 mg/kg respectively daily for 28 days. On day 29th Group I to IV were sacrificed under diethyl ether and blood was collected by cardiac puncture.
| Group | Number of mice of either sex | Treatment (Acetamiprid dissolved in distilled water) | Route |
|---|---|---|---|
| I | 5+5 | Control | Oral |
| II | 5+5 | 10 mg/kg | Oral |
| III | 5+5 | 20 mg/kg | Oral |
| IV | 5+5 | 40 mg/kg | Oral |
Table 1: Experimental design.
Body weight
Body weight of mice in all the groups was recorded at weekly intervals using electronic balance.
Clinical signs
Clinical signs were noted daily once by cage side observation and detailed observation were recorded on weekly basis. Behavioral changes, signs of difficult breathing or prolonged micturation and all signs of toxicity were recorded. Daily once all groups were observed for morbidity and mortality.
Hematological studies
Collection of blood: Blood was collected by heart puncture by using dissociative anesthetic, ketamine (5% Ketamine Hydrochloride) at 22 mg/kg body weight intramuscularly (CPCSEA Guidelines for laboratory animal facility, 2003) on thigh region 20 min before the sacrifice of the mice on respective days. The blood samples were aliquoted for further estimation of Hb, PCV, TLC, TEC, MCV, MCHC, MCH and DLC with anticoagulant. And for biochemical analysis blood was kept in test tube without anticoagulant.
Estimation of hemoglobin: Hemoglobin (Hb) was estimated by Hellige Sahlis hemoglobinometer and expressed in gm%. First the alkaline haematin was formed by N/10 NaOH followed by formation of acid hematin with the help of N/10 HCL as suggested by Cannan [3]. The intensity of color was compared by Hellige Sahlis hemoglobinometer.
Estimation of total erythrocyte count: Total erythrocyte count (TEC) was enumerated by hemocytometer as per standard method of Schalm et al. [4] and was expressed in terms of millions per cubic millimeter (106/cu.mm).
Estimation of total leukocyte count: Total leukocyte count (TLC) was enumerated by hemocytometer as per standard method of Schalm et al. [4] and was expressed in terms of thousands per cubic millimeter (103/cu.mm).
Estimation of packed cell volume: Packed call volume (PCV) was estimated in Wintrobe’s hematocrit tube as per Schalm et al. [4]. Readings were taken after centrifuging at 3000 rpm for 30 min. it was calculated in %.
Estimation of mean corpuscular volume: MCV was expressed by the ratio of percentage of PCV with TEC/cumm.
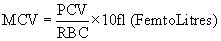
Estimation of mean corpuscular hemoglobin: MCH was estimated by the formula
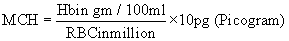
Estimation of mean corpuscular hemoglobin Concentration: MCHC was estimated by the formulas
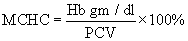
Estimation of differential leukocyte count: Different leukocyte count (DLC) was estimated by Jain [5] and the values were expressed as percentage.
Biochemical studies
Separation of serum: The test tube containing blood serum separation was kept undisturbed in a slanting position. Then after allowing clotting completely, the serum was separated from the clot by using a Pasteur pipette and it was centrifuged to obtain a clear serum. The serum was transferred to screw capped vials and preserved at -20°C till further use.
Estimation of serum glutamate pyruvate transaminase (SGPT): The serum Glutamate pyruvate transaminase (SGPT/ALT) was estimated spectrophotometrically by 2, 4-Dinitrophenyl hydrazine (DNPH) method [6] mentioned in diagnostics kit and the result was expressed in IU/L.
Estimation of serum glutamate oxaloacetate transaminase (SGOT): The serum Glutamate oxaloacetate transaminase (SGOT/ AST) was spectrophotometrically by 2, 4-Dinitrophenyl hydrazine (DNPH) method [6] mentioned in diagnostics kit and the result was expressed in IU/L.
Estimation of alkaline phosphatase (ALP): The alkaline phosphatase (ALP) activity was measured by the method using 4-amino antipyrine, prescribed by Kind and King [7]. Alkaline phosphatase from serum converted phenyl phosphatase in inorganic phosphate and phenol at pH 10.0. Phenol so formed reacted in alkaline medium with 4-amino antipyrine in presence of the oxidizing agent potassium ferricyanide and formed an orange-red colored complex, which was measured calorimetrically at 510 nm. The optical density of the colored complex was proportional to the enzyme activity, which is expressed as IU/L.
Statistical analysis
The results were analyzed by using the Graph pad Inspat3. Software SPSS (Snedechor [8]) (version 10.0) used for statistical analysis.
Body weight
The average weekly body weight (g) of mice recorded in different Groups have been summarized and presented in table 2. There was no significant reduction in body weight of male mice up to 7th day. In Group II there was no significant alteration in body weight up to 14th day of treatment. However the average body weight in Group II gradually declined after 21 days post treatment. And the Group III, receiving 20 mg/kg body weight had their initial average body weight within very little variations with other Groups. And the average body weight steadily increased up to 21st day then decreases on 28thday post treatment.
| Group | 0 day | 7th day | 14th day | 21st day | 28th day |
|---|---|---|---|---|---|
| I (Control) | 24.07x ± 0.202 | 28.93x ± 0.361 | 33.19x ± 0.502 | 34.45abx ± 0.217 | 38.32ax ± 0.528 |
| II | 24.15x ± 0.252 | 28.00x ± 0.397 | 32.46x ± 0.329 | 27.30bcy± 0.322 | 26.37cy ± 0.308 |
| III | 24.21x ± 0.291 | 29.03x ± 0.377 | 30.10y ± 0.363 | 26.47cy ± 0.287 | 24.21dz ± 0.306 |
| IV | 25.01x ± 0.416 | 27.13y ± 0.406 | 26.84z± 0.310 | 21.86cz ± 0.557 | 18.27dz1 ± 0.358 |
Table 2: Comparison of weekly body weight (gm) in male mice of different experimental groups.
The Group IV receiving a dose of 40 mg/kg body weight showed reduction in body weight from 14th day onwards followed with most significant reduction from 21stonwards.
There was no significant reduction in body weight of male mice up to 7th day. Significant (p<0.01) decrease in body weight was observed in mice of Group IV from 14th day onwards when compared with the control Group. However the most significant (p<0.01) dose dependent reduction was observed on 21stday post treatment in Group IV (21.86 ± 0.557) followed by Group III (26.47 ± 0.287) and Group II (27.30 ± 0.322) when compared with Group I (34.45 ± 0.217). And also the result at 28th day post treatment in Group IV (18.27 ± 0.358) followed by Group III (24.21 ± 0.306) and Group II (26.37 ± 0.308) male mice when compared with Group I (38.32 ± 0.528) control mice.
The findings of dose dependent significant decrease in body weight of male mice in the present study correlated with the findings of El – Shahawy et al. [9] treated with acetamiprid and their mixtures with lead acetate and cadmium acetate. Zhang et al. [1] in male mice treated with acetamiprid. However Jain et al. [5] in male wister rats treated with imidacloprid reported no effect on body weight in all treated groups.
Clinical signs
All the experimental mice were closely observed for development of clinical symptoms throughout the experimental period. Mice treated with different doses showed various types of clinical symptoms. Group IV mice receiving highest dose (40 mg/kg body weight) of acetamiprid showed tendency to mouth smacking and salivation, sitting in the corner, depression, reduced feed intake, loose feces, yellow color of urine, respiratory depression etc. However in Group II and Group III mice, except depression, no other clinical signs were observed.
The clinical signs like respiratory depression, profuse salivation, smacking were also observed by Mondal [10] in female rats given orally acetamiprid. The clinical signs observed in the present study were also observed by Cox [11] and Bhardwaj et al. [12] in wistar rats given imidacloprid orally.
Hematological studies
Hemoglobin (Hb): The details of mean ± S.E. values of hemoglobin concentration (g/dl) of all the treatment Groups at 28th day interval are summarized and presented in table 3. The level of significance (p<0.01) in mean hemoglobin concentration between dose dependent Groups showed significant decrease on day 28th post treatment in Group II (13.87 ± 0.344) followed by Group III (12.41 ± 0.191) and Group IV (10.44 ± 0.260) when compared with Group I (14.22 ± 0.158) control mice.
| Parameters | Group | 28 Days |
|---|---|---|
| Hb (g/dl) | I | 14.22 ± 0.158a |
| II | 13.87 ± 0.344a | |
| III | 12.41 ± 0.191b | |
| IV | 10.44 ± 0.260c | |
| PCV (%) | I | 43.66 ± 0.279a |
| II | 42.36 ± 0.352b | |
| III | 41.13 ± 0.285c | |
| IV | 34.85 ± 0.190d | |
| TEC (millions/cumm) | I | 10.16 ± 0.205a |
| II | 9.31 ± 0.247b | |
| III | 4.54 ± 0.341c | |
| IV | 3.81 ± 0.238c | |
| TLC (thousand/cumm) | I | 9.51 ± 0.229a |
| II | 8.84 ± 0.215b | |
| III | 7.08 ± 0.136c | |
| IV | 4.30 ±0.143d | |
| MCV (fl) | I | 87.30 ± 0.337d |
| II | 89.29 ± 0.296c | |
| III | 93.06 ± 0.201a | |
| IV | 91.94 ± 0.263b | |
| MCHC (g/dl) | I | 33.12 ± 0.140a |
| II | 28.83 ± 0.181c | |
| III | 30.57 ± 0.133b | |
| IV | 30.54 ± 0.291b | |
| MCH (pg) | I | 23.53 ± 0.178c |
| II | 24.52 ± 0.292b | |
| III | 28.58 ± 0.162a | |
| IV | 28.04 ± 0.153a |
Table 3: Effect of acetamiprid on hematological profile in mice after daily oral administration for 28 days.
Packed cell volume (PCV): Packed cell volume (%) when determined, the mean values from all the groups revealed a decrease trend with highest dose levels of administration. The significant (p<0.01) decrease on 28thday post treatment in Group II (42.36 ± 0.352) followed by Group III (41.13 ± 0.285) and Group IV (34.85 ± 0.190) as compared with control Group I (43.66 ± 0.279) and it is presented in table 3.
Total erythrocyte count (TEC): The Mean ± S.E. values of TEC (million/cu.mm) of all the Groups at 28th day post treatment are summarized and presented in table 3. And revealed dose dependent significant (p<0.01) decrease as compared to control. The decrease were observed like control Group I (10.16 ± 0.205) mice, significant decrease in mean values of Group II (9.31 ± 0.247) followed by Group III (4.54 ± 0.341) and Group IV (3.81 ± 0.23).
Mean corpuscular volume (Femto litre): The MCV showed variations without any definite dose dependent trend. However the level of significance (p<0.01) was apparent with Group II (89.29 ± 0.296) followed by Group IV (91.96 ± 0.263) and Group III (93.6 ± 0.201) when compared with control Group (87.30 ± 0.337) and represented in table 3.
Mean corpuscular hemoglobin concentration (g/dl): Narrow range of variations with p<0.01 levels of significance was observed in all the Groups when compared with control Group and presented in table 3. The Group II (28.83 ± 0.181) was followed by Group IV (30.54 ± 0.291) and Group III (30.57 ± 0.133) when compared with control Group (33.12 ± 0.140).
Mean corpuscular hemoglobin (pg): Large variations with significant (p<0.01) mean ± S.E. values were found in the treatment Groups and presented in table 3. With Group II (24.52 ± 0.292) followed by Group IV (28.04 ± 0.153) and Group III (28.58 ± 0.162).
In the present study, significant decrease in total leukocyte count (thousand/cu.mm) was in agreement with the previous observation made by Mondal et al. [13] in female rats after oral administrations of acetamiprid. However, El – Shahawy et al. [9] in male albino mice after sub-lethal doses of acetamiprid, lead acetate, and cadmium acetate observed increase in white blood cells. However Bhardwaj et al. [12] found no changes in WBC after oral administrations of imidacloprid at various dose levels for 90 days.
Reduction in the levels of TEC, Hb was also reported earlier by Mondal [10] in acetamiprid treated female rats. Though there were no significant differences observed in mean values of PCV, MCV, MCH, MCHC, in treated groups. Chakraborty [14] observed no statically significant changes in Hb, RBC levels, in goats following consecutive daily oral administration at the rate of 18.48 and 9.24 mg/kg body weight.
The present study of decrease in Hb, PCV, and TEC as observed. Thus it indicating that the insecticide could produce macrocytic hyperchromic anemia.
Total leukocyte count (TLC): Details of mean ± S.E. values of total leukocyte count (thousands/cu.mm) of all the treatment Groups at 28th day are summarized and presented in table 3.
The value of TLC revealed dose dependent significant (p<0.01) decrease on 28th post treatment as compared to control.
However TLC decreased with the respective treatment Groups, from Group II (8.84 ± 0.215) to Group III (7.08 ± 0.136) and Group IV (4.30 ± 0.143) as compared with control Group I (9.51 ± 0.229).
Biochemical studies
Serum glutamate pyruvate transaminase (SGPT/ALT): The study revealed highly significant (p<0.01) dose dependent elevation of SGPT (IU/L) levels in all the treatment Groups at 28th day and the detail of mean ± S.E. values of SGPT of different treatment Groups are presented in table 4. The mean serum Glutamate pyruvate transaminase dose dependent significant (p<0.01) increase in Group II (40.60 ± 0.274) was followed by Group III (69.00 ± 0.150) and Group IV (76.66 ± 0.194) when compared with the values of Group I (30.53 ± 0.376) control mice.
| Parameters | Groups | 28 days |
| ALT (IU/L) | I | 30.53 ± 0.376d |
| II | 40.60 ± 0.274c | |
| III | 69.00 ± 0.150b | |
| IV | 76.66 ± 0.194a | |
| AST (IU/L) | I | 147.09 ± 0.154c |
| II | 168.24 ± 0.537c | |
| III | 198.98 ± 0.310b | |
| IV | 266.16 ± 20.08a | |
| ALP (IU/L) | I | 63.37 ± 0.206d |
| II | 97.65 ± 0.118c | |
| III | 139.19 ± 0.220b | |
| IV | 191.60 ± 1.87a |
Table 4: Effect of acetamiprid on biochemical parameters in serum of mice after daily oral administration for 28 days.
Serum glutamate oxaloacetate transaminase (SGOT/AST): It was determined in IU/L values at 28 days of administration. The mean serum Glutamate Pyruvate transaminase values revealed dose dependent significant (p<0.01) increase was observed in Group II (168.24 ± 0.537) followed by Group III (198.98 ± 0.310), and Group IV (266.26 ± 20.08) as compared with control Group (147.09 ± 0.154) mice. Details of mean ± S.E. values of SGOT of different treatment Groups are presented in table 4.
Alkaline phosphatase
Details of mean ± S.E. values of SGOT of different treatment Groups are presented in table 4. The post administered values at 28th days for all the treatment Groups of alkaline phosphatase (IU/L) was determined including Control Group. A highly significant (p<0.01) mean ± S.E. values with increasing trend was observed in Group II (97.65 ± 0.118) followed by Group III (139.19 ± 0.220) and Group IV (191.60 ± 1.877) when compared with control Group (63.77 ± 0.206) mice.
The findings in the present study correlated with findings of Mondal (2007) in oral administration of acetamiprid to female wister rats, Chakraborty [14] found the above in acetamiprid oral administration in goat. Zhang et al. [1] confirmed significant increase in activity of serum Alanine Transaminase (ALT) of male mice in acetamiprid toxicity. Bhardwaj et al. [12] reported elevation in GPT in imidacloprid toxicity in female rats. The present findings of increase in the value of AST was in agreement with the findings of Bhardwaj et al. [12] in female rats following administration of Imidacloprid and Zhang et al. [1] in male mice following administrations of acetamiprid. Increase in Alkaline phosphatase value was also reported by other workers such as acetamiprid toxicity in female rats [13], acetamiprid in male mice Zhang et al. [1], dichlorovos in male mice [15]. The increase in ALP usually occurred due to its increased synthesis due to damaged liver conditions [16]. Elevated plasma ALP might be due to acute hepatocellular damage and destruction of epithelial cells in gastrointestinal tracts [17].
In the present study, it was found that acetamiprid administered @ 10 mg/kg body weight (1/20th of LD50 value) proved to be non-toxic for the experimental animals under test [18].
The authors are thankful to Hon’ble Vice-Chancellor, West Bengal University of Animal and Fishery Sciences, Kolkata, India for providing the necessary facilities to carry out this research work.
The original research work is dedicated to West Bengal University of Animal and Fishery Sciences, Kolkata, India.