Fungal Genomics & Biology
Open Access
ISSN: 2165-8056
ISSN: 2165-8056
Review - (2024)Volume 14, Issue 1
Cordyceps is diverse genus of the entomopathogenic fungi that have numerous health benefits. It is grown in many parts of the Eastern Asia and its cultivation and popularity is increasing day by day. It is considered one of the three tonics in a traditional Chinese medicine treasury together with ginseng and pilose antler. Cordyceps militaris is the main species whose cultivation has been achieved on artificial media. It has many useful metabolites viz., cordycepin, polysaccharides, ergosterol, ergothioneine, trehalose and mannitol. It can be grown on specific media and the secondary metabolites content can be increased through environment and media manipulation. The standardization of laboratory cultivation has paved the way for its commercial production. However, the biggest hurdle is the strain degeneration. The strains lose their potency and the ability to produce high content of beneficial compounds when sub cultured for several generations. In this review, benefits of Cordyceps, active ingredients isolation and extraction, pathway of Cordycepin biosynthesis, information about Cordyceps related fungi and the recent advancements in the cultivation and strain degeneracy of Cordyceps are discussed.
Ophiocordyceps sinensis; Cordyceps militaris; Cordycepin; Growth media; Strain degeneracy
3’-dAMP: 3’-deoxy Adenosine Monophosphate; 3’-dATP: 3’-deoxy Adenosine Triphosphate; ADSS: Adenyl Succinate Synthetase; AK: Adenosine Kinase; AMPK: Adenosine Monophosphate Protein Kinase; CCs: Cultivated Cordyceps; CE: Capillary Electrophoresis; CMP18: Cordyceps militaris 18-kDa Protein; DF: Developed Fruiting body; FFAs: Free Fatty Acids; GC: Gas Chromatography; GPs: Glycolipids; guaA: Guanosine monophosphate synthetase; GUK: Guanylate Kinase; hENT1: Human Equilibrative Nucleoside Transporters 1; HY: Hyphae; IMPDH: Inosine 5'-Monophosphate Dehydrogenase; LC: Liquid Chromatography; MAT: Mating-Type; MF: Mature Fruiting; MFS: Major Facilitator Superfamily; MG: Monosodium Glutamate; MS: Mass Spectrometry; NCs: Naqu; NDPK: Nucleoside Diphosphate Kinase; NMR Spectrometry: Nuclear Magnetic Resonance Spectrometry; PR: Plumule Rice; PRm: Primordium; SGT1: Suppressor of G2 allele of SKP1; SLs: Sphingolipids; ST: Sclerotium; STZ: Streptozotocin; UV: Ultra Violet; W: Wheat; YE: Yeast Extract; YF: Young Fruiting body
Cordyceps Fr. (L.) is a large and diverse genus of the family Cordycipitaceae (Hypocreales, Ascomycota) comprising more than 625 species according to Myco Bank [1]. They are parasitic fungi, mostly endoparasitoids of insects and other arthropods, while some of them are parasitic on other fungi [2]. Cordyceps genus includes many insect parasites and fungal parasites such as the tongue core club (Cordyceps ophioglossoides) which lives on deer truffles (Elaphomyces spp.) and plant parasites such as the grass kernel fungus (Epichloë typhina). The ergot fungus (Claviceps purpurea), Cordyceps militaris, Ophiocordyceps sinensis (O. sinensis, caterpillar fungus), Cordyceps nutans and Paecilomyces tenuipes are related species. Recently, several new Ophiocordyceps species parasitizing on a range of insect hosts have been identified, e.g., O. asiana and O. tessaratomidarum, O. flavida, O. mizoramensis, O.vespulae, O. bidoupensis, O. hydrangea, O. buquetii, O. laotii and O. puluongensis [3-9]. Recent changes in the nomenclature and modification in classifications of Cordyceps and related genera has been discussed by Shrestha, et al. [10].
Among these medicinal fungi, the most valued one in Chinese medicine is O. sinensis (formerly Cordyceps sinensis), O. sinensis mainly infects pupae but sometime larvae of insects. The healing powers of O. sinensis are documented in Chinese herbal books that are up to 2,000 years old. Another less explored Cordyceps species similar to O. sinensis, C. militaris and C. nutans is found in Japan, Taiwan and China. C. nutans is entomoparasitic ascomycete of hemipteran insects [11]. One more medicinal mushroom Paecilomyces is an anamorph of Cordyceps [12]. P. tenuipes had been used traditionally as an effective, nutritious health food and medicine in China [13,14].
There are many other Cordyceps species that grow on different kind of insects, infect their bodies and the ascomata emerges out from their body especially head to spread the spores. These species mostly infect only on host species thus widespread damage to different species is not possible by one fungus. It is a mechanism in nature that controls the unregulated growth of any species in check since the abundant species has higher chance of getting infected by a parasitic Cordyceps spore. Some of the Cordyceps species also show mind control behavior on their host e.g., Ophioglassus unilateralis after infecting host rainforest carpenter ant, Camponotus leonardi leads it to climb on a tree and bite the midrib on the lower side of a leaf to remain stuck in that position even after its death. This facilitates the dispersal of spores and infection of ants travelling on the ground.
O. sinensis (known as Dong-Chong Xia-Cao in Chinese means ‘worm in winter and grass in summer’) grows better in alpine areas (3000 to 5000 meters high) of Tibet, Chinese and Indian territory which are covered with snow in winter and become grassy in summer. It infects a range of host insects especially Hepialus/Thitarodes (Lepidoptera) [15,16]. Cattle dig and eat it up during summers in the grassy lands to become energetic. Overharvesting of O. sinensis has led to its categorization as endangered species in China (State Order of Chinese Government). O. sinensis fruiting body has been cultivated commercially recently (some patents filed by companies). Mycelial forms have also been cultivated in vitro [17,18].
Cordyceps species are not only recommended as an aphrodisiac (called Himalayan Viagra), but also for strengthening the lungs, kidneys and sperm production, against cough, cold and bleeding. Infected pupae and caterpillars are in great demand as medicines but are not so common in nature, thus the pupae of the silkworm (Bombyx mori) are artificially infected with the fungi to meet the demand. The fruiting bodies harvested in nature may contain high amounts of Arsenic and other heavy metals therefore their sale is stringently regulated by Chinese government since 2016 [19]. The fungus germinates in the living larva, kills and mummifies it. Sometimes, many species including O. sinensis simultaneously infect the insect body. A dark brown stalk-like fruiting body, ascus which is 1-8 cm long, club-shaped orange/red and cylindrical, emerges from the head of insect corpse and stands upright. The clubs are whitish to pale orange inside. Shape and size of ascomata vary widely based on the size of the host, number of stromata formed and on the substrate of mycelial growth [20]. The perithecia are present inside clubs which is covered with the stroma. Its spores are long, filiform, smooth, hyaline and often septate that disintegrate later into smaller 3-7 μm × 1-1.2 μm subspores.
C. militaris is considered as a cheaper substitute for the rare fungus O. sinensis and ranks top among the commercialized Cordyceps species, followed by O. sinensis [21]. C. militaris have higher content of cordycepin as compared to O. sinensis. The consumption of C. militaris have a more potent anti-tumor effect than O. sinensis at the same dose level [22] (Figure 1). Fruiting bodies obtained from cultured C. militaris are sold as health food product and drug material across South East Asia and China [23]. C. militaris is widely distributed in Europe, North and South America and Asia and have multiple medicinal properties [24-26]. Many bioactive compounds have been isolated from it such as cordycepin 3’-deoxyadenosine Monophosphate (3’-dAMP), Cordyceps polysaccharides, ergosterol, ergothioneine trehalose, mannitol etc., [27-29] (Figure 2). Its nutraceuticals are consumed either as culinary mushrooms or in several other forms, e.g., extracts, fermented powder and tinctures. C. militaris have antioxidant, antimicrobial, anti-inflammatory, hypolipidemic, hypoglycemic, antiviral, antimalarial, prosexual, neuroprotective and immuno-protective and anti-tumorigenic [30-32] (Figure 1). In diabetic rat models (induced with Streptozotocin (STZ)), C. militaris treatment improved the sexual parameters e.g. serum testosteron levels, copulatory behaviour, epididymal sperm count and mobility [33]. Aqueous extracts of C. militaris show hypoglycemic activity i.e. anti-Protein Tyrosine Phosphatase 1B (PTP 1B) activity due to the presence of four cerebrosides including cordycerebroside B and a disaccharide [34]. It is also used for stopping bleeding, reducing phlegm, hypnotizing, calming, benefiting liver and kidney, tonifying deficiency, relieving asthma and skin cosmetics [35-39].
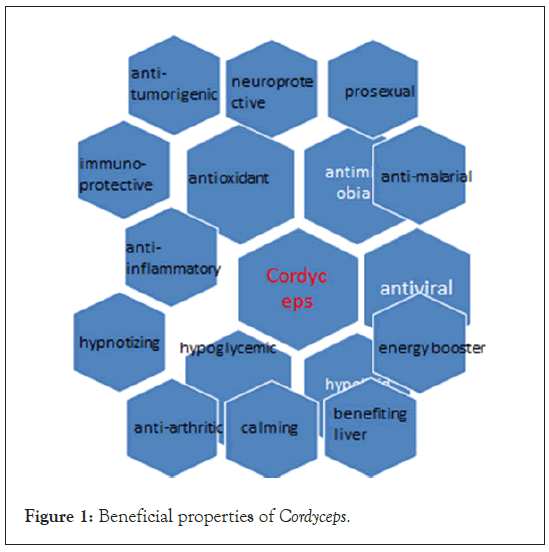
Figure 1: Beneficial properties of Cordyceps.
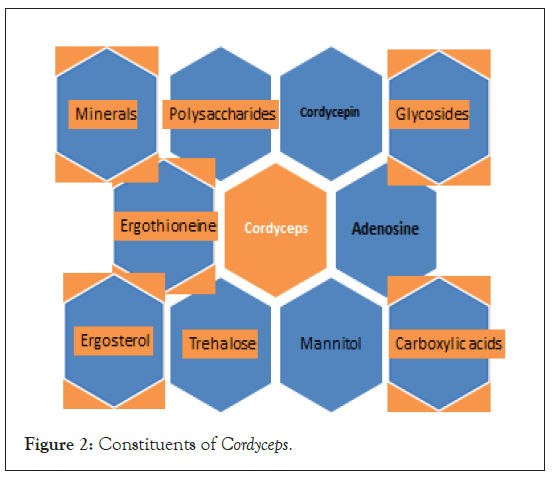
Figure 2: Constituents of Cordyceps.
Active ingredients
Many components have been found from natural and cultured Cordyceps by Liquid Chromatography (LC), Gas Chromatography (GC), Nuclear Magnetic Resonance (NMR) spectrometry and Capillary Electrophoresis (CE) combined with Mass Spectrometry (MS) and UV-visible spectrophotometry such as cordycepin, adenosine, proteins, amino acids, carbohydrates, carboxylic acids, lipids, glycosides and minerals [40,41]. Among them, cordycepin is considered as the most valuable and economically important compound produced by this fungus (Figure 3). It is used to inhibit the growth of bacterial, fungal and viral pathogens including Clostridium paraputrificum and Clostridium perfringens, Bacillus subtilis, Candida, human poliovirus, tobacco mosaic virus and hepatitis C virus etc., [42-47]. In addition to this, cordycepin exhibits insecticidal effects. Cordycepin can induce cell death in pests, including Plutella xylostella, Trypanosoma brucei and Trypanosoma evansi [48-50]. Cordycepin induces programmed cell death in-vivo in P. xylostella. In T. evansi infected mice, a combination of cordycepin (2 mg/kg) and pentostatin (0.2 mg/kg) showed significant therapeutic potential and decreased toxicity. In addition, cordycepin also exhibited antifungal activity e.g. against different Candida isolates (Figure 3). Cordycepin has normally been detected by High-Performance Liquid Chromatography (HPLC), but a new method developed recently used Matrix-Assisted Laser Desorption Ionization-Mass Spectrometry (MALDI-MS) to detect its concentration [51]. From another species, O. sinensis identified 8,541 putative antioxidant peptides using a high-throughput method [52].
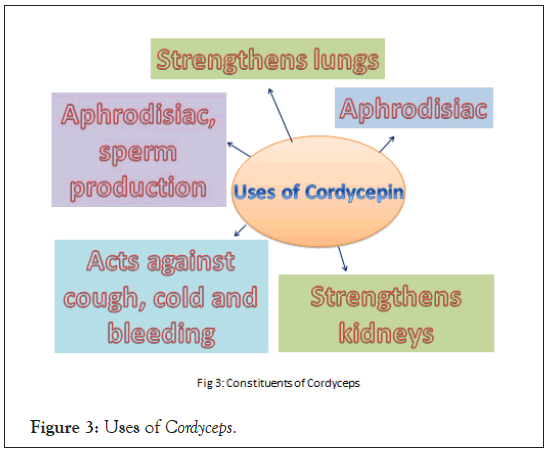
Figure 3: Uses of Cordyceps.
Mechanism of action of active ingredients
Cordycepin is supposed to enter the cells via the human Equilibrative Nucleoside Transporters 1 (hENT1) and phosphorylated by Adenosine Kinase (AK) to 3’-deoxyadenosine Monophosphate (3’-dAMP). After being phosphorylated twice, 3’-dAMP is converted to the active 3’-deoxyadenosine Triphosphate (3’-dATP) by the concerted action of Adenosine Monophosphate Kinase (AMPK) and Nucleoside Diphosphate Kinase (NDPK) enzymes [53,54]. Cordycepin achieves its function by:
• Forming cordycepin triphosphate through phosphorylation which is erroneously recognized as ATP by target enzymes [55].
• Abnormal purine metabolism through erroneous reading of cordycepin as adenosine
• Cordycepin and/or cordycepin-triphosphate directly activate some protein kinases, e.g. AMPK probably due to an increase in the AMP/ATP ratio.
• Cordycepin and/or cordycepin-triphosphate inhibit targets kinases by replacing ATP binding with the highly structurally similar cordycepin-triphosphate binding and/or the activation of protein phosphatases [56,57].
• Cordycepin and/or cordycepin-triphosphate interrupt mRNA polyadenylation because of the erroneous recognition of cordycepin-triphosphate as ATP by poly A polymerase [58].
Cordycepin can specifically inhibit transcription efficiency and thus RNA elongation and also has the potential to act as a ligand. Recently diastereoisomers of ProTide, a modified form of cordycepin named NUC-7738, was synthesized artificially, which have up to 40 times greater potency for killing cancer cells than the cordycepin [59-61].
Total extracts with water or 50% ethyl alcohol and polysaccharides from C. militaris tend to promote type 1 immunity, whereas that with 70-80% ethyl alcohol promote type 2 immunity. Cordycepin also promotes type 2 immunity. Different immunomodulatory effects of C. militaris extracts obtained using different solvents might be due to the different polarities of the final products.
C. militaris is not only rich in proteins (39.37%; 1.8, 1.5 and 1.9 times of the protein content of pork, beef and mutton) and amino acids, but also in more than 30 trace elements required by human body. The content of Phosphorus (P), Zinc (Zn), Copper (Cu) and Iron (Fe) are respectively 1.8, 2.1, 8.8 and 3.5 times of that of O. sinensis. Additionally, the content of Selenium (Se) is equivalent to that of Astragalus. It contains an 18-kDa protein Cordyceps Militaris Protein-18 (CMP18), which induces apoptosis and might be toxic when the fungi are eaten fresh but gets destroyed upon cooking [62]. CMP18 induces apoptosis via mitochondria-dependent pathway.
Artificial cultivation and enhancement of active ingredients
Cost-effective, cheap, suitable and abundant resources such as by-products of the agricultural and agro-industrial activities are being explored for the development of commercial cultivation of C. militaris and other edible and medicinal mushrooms [63,64]. A lot of literature is available for media optimization and enhancing the active ingredients content.
A wide range of methodologies are used to produce fruiting bodies and mycelia of C. militaris viz. submerged static fermentation, solid-state fermentation, cultivation following larval or pupal infection and one-step non-static or repeated batch fermentation in liquid media [65-72]. There are many media on which C. militaris can be grown. One of the C. militaris culture medium comprises 40-50 g of glucose, 20-30 g of maltose, 5-15 g of beef extract, 5-15 g of peptone, 25-35 g of calcium nitrate, 0.2-0.6 g of magnesium sulfate, 0.2-2.0 g of dipotassium hydrogen phosphate, 0.2-0.6 g of calcium chloride, 30-60 g of silkworm chrysalis powder and 20-50 g of agar per 1000 ml of distilled water. 0.2-1.0 g of common salt per liter is also added (Chinese Patent #132542158). The content of three pharmaceutically important products of C. militaris viz. polysaccharides, cordecepin and adenosine varies with time in fruiting bodies. The use of corn cobs or cottonseed shells to wheat bran and rice at ratios of 8:1:1 (w/w/w) resulted in higher yields of fruit bodies compared to the conventional rice media, while corn cobs produced fruit bodies with the highest cordycepin content. Addition of vegetable oils such as rapeseed, olive, palm, peanut, corn, soybean and sunflower seeds in the static cultures of C. militaris promoted mycelium growth. Moreover, the addition of peanut oil significantly increased cordycepin content. Optimized media formulation for C. militaris contained 20% beef broth, 0.10% peptone, 2% glucose, 0.15% yeast extract, 0.20% KH2PO4 and 0.02% MgSO4. Zhu, et al. [73], found that in C. militaris strain CM-H0810 which grows in the surrounding areas of Shanghai and Guangdong province, the polysaccharide content was highest at 45 d (3.46%), cordycepin at 60 d (3.57 μg/mg) and adenosine at 35 d (1.86 μg/mg). Liang, et al. [74], studied two C. militaris strains, “H” and “L” and obtained the highest yield and biological efficiency with Pearl Barley (PB) substrate for C. militaris H strain and with brown rice+peptone substrate for C. militaris strain L. Furthermore, they observed the highest cordycepin content on Wheat (W)+Monosodium Glutamate (MG) substrate, mannitol content on Plumule Rice (PR)+MG and adenosine content on PR+Yeast Extract (YE) substrates in the C. militaris H fruiting bodies. In C. militaris L, highest cordycepin was reported with W+MG substrate, mannitol with PB substrate and adenosine with PB+YE substrate. In another study, the concentration of bioactive compounds was compared between C. militaris and(i) Fruiting bodies on wheat; (ii) Fruiting bodies on pupae; (iii) Sclerotium (the pupae portion) and (iv) Sclerotium with fruiting bodies (stroma). The amounts of cordycepin, carotenoids and superoxide dismutase were found to be higher in the fruiting bodies on pupae than that in the fruiting bodies on wheat, whereas the amounts of adenosine, polysaccharides and mannitol were higher in the fruiting bodies on wheat than in the fruiting bodies on pupae. The adenosine, polysaccharide and mannitol contents in the sclerotium (the pupae portion) with fruiting bodies were significantly lower than those of the fruiting bodies on wheat.
Light and heat stress also influences cordycepin biosynthesis in C. militaris. It was suggested that during the late maturation stage of ascomata, heat and light stresses led to a significant increase in cordycepin biosynthesis without affecting biological efficiency and heat stress significantly promotes carotenoid production [75]. Moreover, it was observed that the optimal growth temperature for C. militaris is 20°C on agar medium, while growth at 25°C is compromised.
Mycelium growth of C. militaris also proved suitable for starch-processing-industry waste under solid-state and submerged culture conditions. High level production of adenosine was reported by Lim, et al. [76], using millet as solid substrate under dark for the first 7 days and harvested on day 40, which decreased till day 50. High cordycepin level was produced by using soybean solid substrate and keeping in the dark for the first 14 days and harvesting on 50th day. In these conditions, Cordycepin increased from day 40 to day 50. Authors also reported a high level of D-mannitol using millet as solid substrate and keeping the culture in dark for first 7 days and harvesting on 50th day. Tao, et al. [77], studied cultivation of six strains of C. militaris on different substrates e.g. rice, wheat and tussah (Antheraea pernyi) pupae and found that different strains grew better on different substrates e.g. strain CM3 showed best efficiency on rice and wheat (62.26% and 54.48% respectively), strain CM9 on tussah pupae (biological efficiency of 291.70%). Further they observed highest adenosine (2.62 mg g-1) content in fruiting bodies of strain CM9 cultivated on tussah pupae and highest cordycepin (5.68 mg g-1) in strain CM4 cultivated on wheat. A high amount of Cordycepin may be itself harmful for the C. militaris, therefore a feedback mechanism to produce pentostatin, an adenosine deaminase inhibitor exists in C. militaris [78]. Hypoxia adaptation by introduction of Vitreoscilla haemoglobin in media, improved the growth, biomass and crude polysaccharides’ content in C. militaris [79]. The efficient cordycepin production was explored using six edible insects as substrates. Highest yield of cordycepin was produced by the cultivation on Allomyrina dichotoma which was 34 times that on Bombyx mori pupae. Fat content of insects was found to be important for cordycepin production. A positive correlation was observed between oleic acid content and cordycepin production. The transcriptional levels of cns1 and cns2, genes involved in cordycepin. biosynthesis, were higher in cordyceps grown on A. dichotoma than on other five tested insects [80].
It is important that cultured fungus should replace the natural one to decrease the burden on natural resources. Wild, O. sinensis takes 1-2 years to complete its life cycle, while cultured, C. militaris takes only 4-6 weeks for fruiting in artificial culture. Artificial cultivation of O. sinensis was difficult but it has been achieved recently [81-84]. In vitro-cultured mycelia of O. sinensis showed only 5% genetic variability from natural counterparts and indicated almost similar D-mannitol content, supporting replacement of the natural fungus with the cultured one [85]. Tang, et al. [86], achieved the successful cultivation of O. sinensis fruiting body with high mycelial protein content of 2.11% by media optimization. Some companies (e.g. Aloha Medicinals, USA) have filed patents for O. sinensis cultivation methods. However, there are conflicting reports-whether these have optimized the process. Kaushik, et al. [87], reported higher amount of cordycepin with growth supplements: nucleosides hypoxanthine and adenosine; amino acids-glycine and glutamine; plant hormones-1-Naphthaleneacetic Acid (NAA) and 3-Indoleacetic Acid (IAA); vitamin-thiamine (B1) in O. sinensis.
Biosynthesis pathway of C. militaris contains four genes cns1, cns2, cns3 and cns4 (Figure 4). Genes cns1, cns2 and cns3 for cordycepin synthesis are present on adjacent loci. Functional verification of the cns1 and cns2, genes essential for cordycepin production was confirmed by generating Aspergillus nidulans knockout mutants and the heterologous gene expression in Saccharomyces cerevisiae and Metarhizium robertsii. Similarly, functional verification of the cns3 for pentostatin production was achieved through heterologous expression of cns3 in M. robertsii and C. bassiana. Cns1 and Cns2 protein interaction was also proved by yeast two-hybrid and co-localisation-studies. Agrobacterium tumefaciens mediated transformation protocol for O. sinensis was standardized by co-cultivation of fungal recipient strains [88]. Pathways in C. cicadae, C. kyushensis and alternative pathway in C. militaris have also been deciphered recently [89-92].
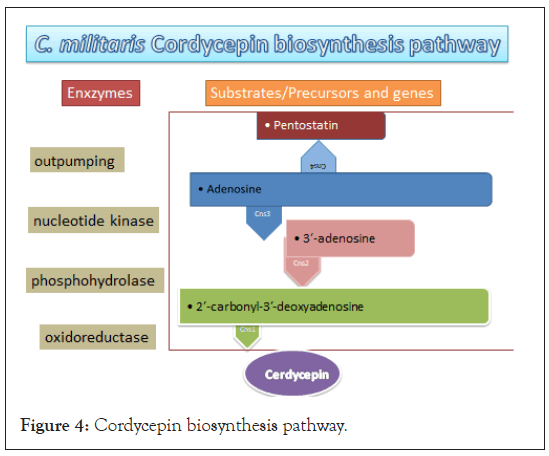
Figure 4: Cordycepin biosynthesis pathway.
Phenotypic degeneration during artificial cultivation
Phenotypic degeneration is a significant problem for mushroom growers. Usually fungi lose their virulence and their morphology is altered when they are repeatedly sub-cultured on artificial media. Morphological changes include colour and growth form modifications and reduced sporulation [93]. Various terms have been used to describe this phenomenon including phenotypic phenotypic degeneration, instability or deterioration, dual phenomenon, saltation and attenuation [94,95]. Particularly in C. militaris, degenerated cultures demonstrate longer growth cycle, slower mycelial growth, a lighter colour of hyphae (probably due to decrease in pigment content), reduced (or no) ability to produce fruit bodies, abnormal fruit body formation, decrease in the number of primordia, decrease in conidia production, decrease in secondary metabolites content, lower dehydrogenase activity and reduced cellulase and amylase activities, as well as lower extracellular and higher intracellular polysaccharides content [96-101]. It becomes more evident after fourth or fifth generations. Several factors that are either intrinsic/genetic or cultivation-related are involved in C. militaris degeneration. Developing suitable methodologies and interventions to avoid (or minimize) culture degeneration, improving ascomata and mycelia production and/or on enhancing cordycepin output by adopting various approaches and the use of both solid and liquid substrates are important research problems currently pursued in this field [102-111]. Beside this, use of breakthrough technologies such as genetic engineering can considerably improve C. militaris biomass content and various metabolites, including cordycepin [112-114]. A recent study standardized the Clustered Regularly Interspaced Short Palindromic Repeats-Cas9 (CRISPR-Cas9) gene editing technique for C. militaris [115].
He, et al. [116], determined that the mineral elements K+, Ca2+ and Zn2+ (at concentrations of 1.0 g L−1, 0.02 gL−1 and 250-375 μgL−1 respectively) delayed the degeneration of different types of C. militaris and whereas Mn2+ and Mg2+ (at trace concentrations) promoted the degeneration. Degeneration of C. militaris was also affected by oxidative stress. Furthermore, homokaryosis promoted degeneration of C. militaris. The degenerated strains could be rejuvenated by cross-mating of their single ascospore isolates.
Molecular mechanisms of phenotypic degeneration
Molecular mechanisms that affect C. militaris culture degeneration are not much understood. Continuous culture induced Mating-Type (MAT) loci allele segregation is proposed to be one mechanism responsible for culture degeneration [117-120]. DNA methylation and gene mutation are also considered to be responsible for strain degeneration. A transcriptome-wide study during C. militaris sub culturing found that the genes involved in stress response such as the production of streptothricin acetyltransferase, glutathione S-transferase, gamma-glutamyl transpeptidase, alcohol dehydrogenase, the 30 kDa heat shock protein, Major Facilitator Superfamily (MFS) multidrug transporter and detoxification were up-regulated during strain degeneration. Similarly, expression of genes involved the metabolism of proteins, amino acids, carbohydrates, lipids, nucleic acids and nucleotides were significantly up-regulated e.g. mitochondrial co-chaperone GrpE, metalloprotease 1, trypsin-like serine protease, Suppressor of G2 allele of SKP1 (SGT1) and Cysteine and Histidine-Rich Domains (CHORD)-containing Proteins (CS), acetate transporter, formyl tetrahydrofolate deformylase, nucleoside triphosphate hydrolases and uracil phosphoribosyl transferase. On the basis of these findings, authors proposed that the strain degeneration mechanism in C. militaris was associated with genes involved in Deoxyribonucleic Acid (DNA) methylation, toxin biosynthesis, energy metabolism and chromosome remodeling.
Transcriptomics, metabolics and lipidomics
Recent Omics studies have significantly improved our understanding of various aspects of Cordyceps fungi [121-125]. Transcriptomics of wild type and cultivated O. sinensis showed that the fatty acid metabolism was more active in wild O. sinensis compared to cultivated O. sinensis, evidenced by downregulation of genes encoding various enzymes involved in fatty acids metabolism e.g. acyl Coenzyme A (acyl- CoA) dehydrogenase, enoyl-CoA hydratase, 3-ketoacyl-CoA thiolase and acetyl-CoA acetyltransferase in cultivated O. sinensis. Liquid Chromatography Mass Spectroscopy (LC-MS)/MS-based metabolomics analysis in O. sinensis from Naqu (NCs) and Yushu (YCs), infected-stiff worm and their substituents i.e. artificially Cultivated Cordyceps species (CCs) and mycelia, identified 901 metabolites. The contents of adenosine and cordycepin were significantly higher in cultivated O. sinensis than in the natural types whereas the levels of mannitol and polysaccharides were lower. However, the contents in the stiff worms were not significantly different from those in natural ones. Mannitol was present maximally in natural types followed by cultivated types and stiff worm. An integrated metabolomics and transcriptomics analysis of O. sinensis suggested the involvement of Inosine 5'-Monophosphate Dehydrogenase (IMPDH), Adenyl Succinate Synthetase (ADSS), Adenosine Kinase (AK), Guanylate Kinase (GUK) and guanosine Monophosphate Synthetase (guaA) genes in the synthesis of purine nucleotides and nucleosides.
Transcript analysis of C. militaris cultivation on germinated soyabean seeds showed that the gene expression in the early stages of fungus development is important for cordycepin biosynthesis and isoflavone modification metabolic pathways. It further revealed that transcription profile changed after two weeks of incubation. Based on a transcriptomics study in C. militaris, Zhang, et al. [126], proposed that sclerotium showed increased oxidative stress and energy metabolism and mitogen-activated protein kinase signaling might induce the formation of fruiting body. Furthermore, n_os_milR16, n_os_milR21, n_os_milR34 and n_os_milR90 milRNAs could regulate the induction of fruiting body in it. In a lipidomics study, 435 lipids were detected from O. sinensis (Naqu, NCs and Yushu, YCs) and its substituents i.e. artificially CCs and mycelia from bailing capsules (BLs; fermented Cordyceps). There were less differences among bioactive lipids such as Sphingolipids (SLs), Glycolipids (GPs) and Fatty Acids (e.g., unsaturated Free Fatty Acids (FFA) and eicosanoid) between CCs and wild cordyceps while significant differences existed between BLs and wild Cordyceps. Majority of differentially expressed GPs and SLs were higher in BLs whereas most of differential Free Fatty Acids (FFAs) and eicosanoids were lower in BLs [127]. Li, et al. [128], carried out a transcriptomics study at six developmental stages i.e. Hyphae (HY), Sclerotium (ST), Primordium (PRm), Young Fruiting body (YF), Developed Fruiting body (DF) and Mature Fruiting body (MF) in O. sinensis, PR and MF stages grouped together, suggesting that primordium differentiation and sexual maturation had similar gene expression patterns. However, the ST and HY stages were far apart developmentally evidenced by more Diethylene Glycols (DEGs) between them, covering 47.5% of the genome. A recent study showed the cultivated O. sinensis contained more of amino acids and derivatives, carbohydrates and derivatives and phenolic acids than wild O. sinensis, but lesser contents of most nucleosides and nucleotides. There are other studies also that shed a light on the pathways and active ingredient contents in correlation with different treatments [129-131].
Cordyceps fungus has many species which have proven and potential therapeutic activities. O. sinensis is the most sought after of these, found only in the Himalayas. It is dug by the native people to sell in the high demand market. This process has created a threat to the survival of this valuable species. Therefore, alternative species C. militaris were discovered that could be easily cultivated on the artificial substrates. The active principles in natural and cultivated Cordyceps, responsible for various therapeutic potentials have been compared and being further increased with the help of elicitation. Media manipulations were performed to achieve high bioactive compounds such as cordycepin, mannitol, adenosine, polysaccharides, ergosterol and Superoxide Dismutase (SOD) in C. militaris. Recently cultivation of O. sinensis has also been achieved on artificial media but with good scope for improvement. The action mechanisms of bioactive compounds are intensively being investigated and newer mechanisms are being unfolded. Many companies have intensified their efforts for its commercial exploitation as drug candidate for several diseases for which comprehensive data need to be generated before getting regulatory approvals. Higher accumulation of cordycepin and other active ingredients and cheaper cultivation methods of Cordyceps spp., are the needs of the hour for its commercial exploitation and availability to the larger population.
Author declares that there is no conflict of interest.
Everything about the manuscript was planned and performed by VKD.
The funding for the work was provided by DEI minor project grant to VKD.
No data was generated to share.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed ]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Dalal VK (2024) Action Mechanism, Benefits and Cultivation (ABC) of Cordyceps. Fungal Genom Biol. 14:243
Received: 19-Mar-2024, Manuscript No. FGB-24-30266; Editor assigned: 21-Mar-2024, Pre QC No. FGB-24-30266(PQ); Reviewed: 04-Apr-2024, QC No. FGB-24-30266; Revised: 11-Apr-2024, Manuscript No. FGB-24-30266(R); Published: 18-Apr-2024 , DOI: 10.35248/2165-8056.24.14.243
Copyright: © 2024 Dalal VK. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.